A unique enhancer boundary complex on the mouse ribosomal RNA genes persists after loss of Rrn3 or UBF and the inactivation of RNA polymerase I transcription
- PMID: 28715449
- PMCID: PMC5536353
- DOI: 10.1371/journal.pgen.1006899
A unique enhancer boundary complex on the mouse ribosomal RNA genes persists after loss of Rrn3 or UBF and the inactivation of RNA polymerase I transcription
Abstract
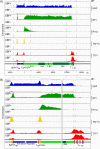
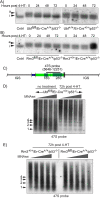
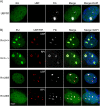
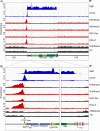
Transcription of the several hundred of mouse and human Ribosomal RNA (rRNA) genes accounts for the majority of RNA synthesis in the cell nucleus and is the determinant of cytoplasmic ribosome abundance, a key factor in regulating gene expression. The rRNA genes, referred to globally as the rDNA, are clustered as direct repeats at the Nucleolar Organiser Regions, NORs, of several chromosomes, and in many cells the active repeats are transcribed at near saturation levels. The rDNA is also a hotspot of recombination and chromosome breakage, and hence understanding its control has broad importance. Despite the need for a high level of rDNA transcription, typically only a fraction of the rDNA is transcriptionally active, and some NORs are permanently silenced by CpG methylation. Various chromatin-remodelling complexes have been implicated in counteracting silencing to maintain rDNA activity. However, the chromatin structure of the active rDNA fraction is still far from clear. Here we have combined a high-resolution ChIP-Seq protocol with conditional inactivation of key basal factors to better understand what determines active rDNA chromatin. The data resolve questions concerning the interdependence of the basal transcription factors, show that preinitiation complex formation is driven by the architectural factor UBF (UBTF) independently of transcription, and that RPI termination and release corresponds with the site of TTF1 binding. They further reveal the existence of an asymmetric Enhancer Boundary Complex formed by CTCF and Cohesin and flanked upstream by phased nucleosomes and downstream by an arrested RNA Polymerase I complex. We find that the Enhancer Boundary Complex is the only site of active histone modification in the 45kbp rDNA repeat. Strikingly, it not only delimits each functional rRNA gene, but also is stably maintained after gene inactivation and the re-establishment of surrounding repressive chromatin. Our data define a poised state of rDNA chromatin and place the Enhancer Boundary Complex as the likely entry point for chromatin remodelling complexes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Moss T, Langlois F, Gagnon-Kugler T, Stefanovsky V. A housekeeper with power of attorney: the rRNA genes in ribosome biogenesis. Cell Mol Life Sci. 2007;64(1):29–49. doi: 10.1007/s00018-006-6278-1 . - DOI - PMC - PubMed
-
- Hinnebusch AG, Ivanov IP, Sonenberg N. Translational control by 5'-untranslated regions of eukaryotic mRNAs. Science. 2016;352(6292):1413–6. Epub 2016/06/18. doi: 10.1126/science.aad9868 . - DOI - PMC - PubMed
-
- Loreni F, Mancino M, Biffo S. Translation factors and ribosomal proteins control tumor onset and progression: how? Oncogene. 2014;33(17):2145–56. Epub 2013/05/07. doi: 10.1038/onc.2013.153 . - DOI - PubMed
-
- Brar GA, Weissman JS. Ribosome profiling reveals the what, when, where and how of protein synthesis. Nat Rev Mol Cell Biol. 2015;16(11):651–64. Epub 2015/10/16. doi: 10.1038/nrm4069 . - DOI - PMC - PubMed
-
- Ingolia NT, Lareau LF, Weissman JS. Ribosome Profiling of Mouse Embryonic Stem Cells Reveals the Complexity and Dynamics of Mammalian Proteomes. Cell. 2011;147(4):789–802. doi: 10.1016/j.cell.2011.10.002 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

