Germline Mutation Status, Pathological Complete Response, and Disease-Free Survival in Triple-Negative Breast Cancer: Secondary Analysis of the GeparSixto Randomized Clinical Trial
- PMID: 28715532
- PMCID: PMC5710508
- DOI: 10.1001/jamaoncol.2017.1007
Germline Mutation Status, Pathological Complete Response, and Disease-Free Survival in Triple-Negative Breast Cancer: Secondary Analysis of the GeparSixto Randomized Clinical Trial
Abstract
Importance: The GeparSixto trial provided evidence that the addition of neoadjuvant carboplatin to a regimen consisting of anthracycline, taxane, and bevacizumab increases pathological complete response (pCR) rates in patients with triple-negative breast cancer (TNBC). Whether BRCA1 and BRCA2 germline mutation status affects treatment outcome remains elusive.
Objective: To determine whether BRCA1 and BRCA2 germline mutation status affects therapy response in patients with TNBC.
Design, setting, and participants: This secondary analysis of a randomized clinical trial used archived DNA samples and cancer family history of 315 patients with TNBC enrolled between August 1, 2011, and December 31, 2012, in the GeparSixto trial. In all, 291 participants (92.4%) were included in this multicenter prospective investigation. DNA samples were analyzed for germline mutations in BRCA1, BRCA2, and 16 other cancer predisposition genes. The pCR rates between the carboplatin and noncarboplatin arms were compared. Genetic analyses were performed at the Center for Familial Breast and Ovarian Cancer in Cologne, Germany; data analysis, November 1 through December 31, 2015.
Main outcomes and measures: Proportion of patients who achieved pCR and disease-free survival after neoadjuvant treatment according to BRCA1 and BRCA2 germline mutation status. For pCR rates, the ypT0/is ypN0 definition was used as a primary end point.
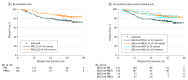
Results: Of the 291 patients with TNBC, all were women; the mean (SD) age was 48 (11) years. The pCR rate in the carboplatin group was 56.8% (83 of 146) and 41.4% (60 of 145) in the noncarboplatin group (odds ratio [OR], 1.87; 95% CI, 1.17-2.97; P = .009). Pathogenic BRCA1 and BRCA2 germline mutations were present in 50 of the 291 patients (17.2%). In the noncarboplatin arm, the pCR rate was 66.7% (16 of 24) for patients with BRCA1 and BRCA2 mutations and 36.4% (44 of 121) for patients without (OR, 3.50; 95% CI, 1.39-8.84; P = .008). The high pCR rate observed in BRCA1 and BRCA2 mutation carriers (16 of 24 [66.7%]) was not increased further by adding carboplatin (17 of 26 [65.4%]). In contrast, carboplatin increased response rates in patients without BRCA1 and BRCA2 mutations: 66 of the 120 patients (55%) without BRCA1 and BRCA2 mutations achieved pCR in the carboplatin arm vs 44 of the 121 patients (36.4%) in the noncarboplatin arm (OR, 2.14; 95% CI, 1.28-3.58; P = .004). Patients without pathogenic BRCA1 and BRCA2 alterations showed elevated disease-free survival rates when carboplatin was added (without carboplatin, 73.5%; 95% CI, 64.1%-80.8% vs with carboplatin, 85.3%; 95% CI, 77.0%-90.8%; hazard ratio, 0.53; 95% CI, 0.29-0.96; P = .04).
Conclusions and relevance: Under the nonstandard GeparSixto polychemotherapy regimen, patients without BRCA1 and BRCA2 germline mutations benefited from the addition of carboplatin and those with BRCA1 and BRCA2 mutations showed superior response rates without additive effects observed for carboplatin.
Trial registration: clinicaltrials.gov Identifier: NCT01426880.
Conflict of interest statement
Figures
Comment in
-
Use of Neoadjuvant Platinum-The Ongoing Conundrum.JAMA Oncol. 2017 Oct 1;3(10):1312-1314. doi: 10.1001/jamaoncol.2017.1954. JAMA Oncol. 2017. PMID: 28715579 No abstract available.
References
-
- von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15(7):747-756. - PubMed
-
- Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33(1):13-21. - PMC - PubMed
-
- Sikov WM, Berry DA, Perou CM, et al. Event-free and overall survival following neoadjuvant weekly paclitaxel and dose-dense AC +/− carboplatin and/or bevacizumab in triple-negative breast cancer: outcomes from CALGB 40603 (Alliance) [abstract S2-05]. Cancer Res. 2016;76(4)(suppl):S2-S5.
-
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938-1948. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

