Treatment of premenstrual dysphoria with continuous versus intermittent dosing of oral contraceptives: Results of a three-arm randomized controlled trial
- PMID: 28715852
- PMCID: PMC5629109
- DOI: 10.1002/da.22673
Treatment of premenstrual dysphoria with continuous versus intermittent dosing of oral contraceptives: Results of a three-arm randomized controlled trial
Abstract
Background: Although traditionally dosed combined oral contraceptives (COCs) (21 days of active pills, 7 days of inactive pills) have not been demonstrated as superior to placebo for the treatment of premenstrual dysphoria (PMD), some randomized controlled trials (RCTs) indicate that oral contraceptives administered with a shortened or eliminated hormone-free interval are superior to placebo. However, results of such trials are mixed, and no existing studies have directly compared continuous and intermittent dosing schedules of the same oral contraceptive. The present study compared placebo, intermittent dosing of oral contraceptives, and continuous dosing of contraceptives for the treatment of PMD.
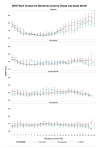
Methods: Fifty-five women with prospectively confirmed PMD completed a three-arm, RCT in which they were randomized to 3 months of placebo (n = 22), intermittent drospirenone/ethinyl estradiol dosed on a 21-7 schedule (n = 17), or continuous drospirenone/estradiol (n = 16) following a baseline assessment month.
Results: All three groups demonstrated similar, robust reductions in premenstrual symptoms over time. A marked placebo response was observed.
Conclusions: The study fails to replicate a uniquely beneficial effect of continuous COC on PMD. Additional work is needed to understand the psychosocial context bolstering the placebo response in women with PMD.
Trial registration: ClinicalTrials.gov NCT00927095.
Keywords: oral contraceptives; premenstrual syndrome; randomized controlled trial.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no conflicts of interest to report.
Figures
References
-
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion: premenstrual syndrome. International Journal of Gynaecology and Obstetrics. 1995:5080–5084. - PubMed
-
- Benedetti F. The placebo response: science versus ethics and the vulnerability of the patient. World Psychiatry. 2012;11(2):70–72. http://doi.org/10.1016/j.wpsyc.2012.05.003. - DOI - PMC - PubMed
-
- Bloch M, Schmidt PJ, Rubinow DR. Premenstrual Syndrome: Evidence for Symptom Stability Across Cycles. American Journal of Psychiatry. 1997;154(12):1741–1746. http://doi.org/10.1176/ajp.154.12.1741. - DOI - PubMed
-
- De la Fuente-Fernández R. Expectation and Dopamine Release: Mechanism of the Placebo Effect in Parkinson’s Disease. Science. 2001;293(5532):1164–1166. http://doi.org/10.1126/science.1060937. - DOI - PubMed
-
- De la Fuente-Fernández R. The placebo effect in Parkinson’s disease. Trends in Neurosciences. 2002;25(6):302–306. http://doi.org/10.1016/S0166-2236(02)02181-1. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical