The Functional Neuroanatomy of Human Face Perception
- PMID: 28715955
- PMCID: PMC6345578
- DOI: 10.1146/annurev-vision-102016-061214
The Functional Neuroanatomy of Human Face Perception
Abstract
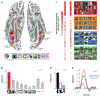
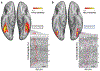
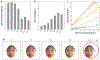
Face perception is critical for normal social functioning and is mediated by a network of regions in the ventral visual stream. In this review, we describe recent neuroimaging findings regarding the macro- and microscopic anatomical features of the ventral face network, the characteristics of white matter connections, and basic computations performed by population receptive fields within face-selective regions composing this network. We emphasize the importance of the neural tissue properties and white matter connections of each region, as these anatomical properties may be tightly linked to the functional characteristics of the ventral face network. We end by considering how empirical investigations of the neural architecture of the face network may inform the development of computational models and shed light on how computations in the face network enable efficient face perception.
Keywords: FFA; fMRI; face network; face recognition; mid-fusiform sulcus; population receptive fields; ventral stream.
Figures
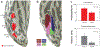


References
-
- Allison T, Ginter H, McCarthy G, Nobre AC, Puce A, et al. 1994a. Face recognition in human extrastriate cortex. J. Neurophysiol 71:821–25 - PubMed
-
- Allison T, McCarthy G, Nobre A, Puce A, Belger A. 1994b. Human extrastriate visual cortex and the perception of faces, words, numbers, and colors. Cereb. Cortex 4:544–54 - PubMed
-
- Allison T, Puce A, Spencer DD, McCarthy G. 1999. Electrophysiological studies of human face perception. I: potentials generated in occipitotemporal cortex by face and non-face stimuli. Cereb. Cortex 9:415–30 - PubMed
-
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K. 2000. Brodmann’s areas 17 and 18 brought into stereotaxic space—where and how variable? NeuroImage 11:66–84 - PubMed
-
- Amunts K, Zilles K. 2015. Architectonic mapping of the human brain beyond Brodmann. Neuron 88:1086–107 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

