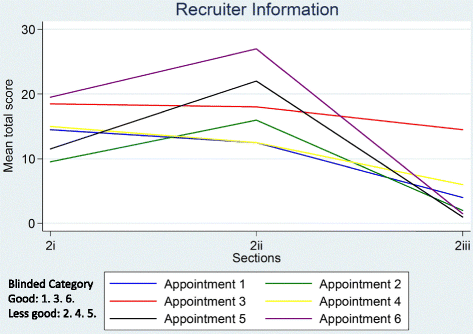
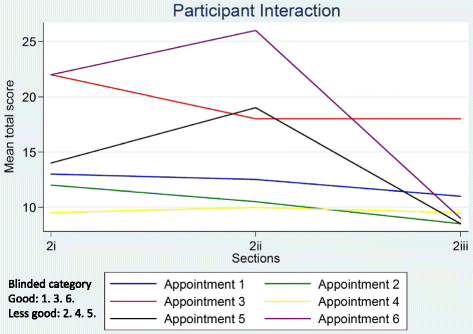
Informed consent in randomised controlled trials: development and preliminary evaluation of a measure of Participatory and Informed Consent (PIC)
- PMID: 28716064
- PMCID: PMC5513045
- DOI: 10.1186/s13063-017-2048-7
Informed consent in randomised controlled trials: development and preliminary evaluation of a measure of Participatory and Informed Consent (PIC)
Abstract
Background: Informed consent (IC) is an ethical and legal prerequisite for trial participation, yet current approaches evaluating participant understanding for IC during recruitment lack consistency. No validated measure has been identified that evaluates participant understanding for IC based on their contributions during consent interactions. This paper outlines the development and formative evaluation of the Participatory and Informed Consent (PIC) measure for application to recorded recruitment appointments. The PIC allows the evaluation of recruiter information provision and evidence of participant understanding.
Methods: Published guidelines for IC were reviewed to identify potential items for inclusion. Seventeen purposively sampled trial recruitment appointments from three diverse trials were reviewed to identify the presence of items relevant to IC. A developmental version of the measure (DevPICv1) was drafted and applied to six further recruitment appointments from three further diverse trials to evaluate feasibility, validity, stability and inter-rater reliability. Findings guided revision of the measure (DevPICv2) which was applied to six further recruitment appointments as above.
Results: DevPICv1 assessed recruiter information provision (detail and clarity assessed separately) and participant talk (detail and understanding assessed separately) over 20 parameters (or 23 parameters for three-arm trials). Initial application of the measure to six diverse recruitment appointments demonstrated promising stability and inter-rater reliability but a need to simplify the measure to shorten time for completion. The revised measure (DevPICv2) combined assessment of detail and clarity of recruiter information and detail and evidence of participant understanding into two single scales for application to 22 parameters or 25 parameters for three-arm trials. Application of DevPICv2 to six further diverse recruitment appointments showed considerable improvements in feasibility (e.g. time to complete) with good levels of stability (i.e. test-retest reliability) and inter-rater reliability maintained.
Conclusions: The DevPICv2 provides a measure for application to trial recruitment appointments to evaluate quality of recruiter information provision and evidence of patient understanding and participation during IC discussions. Initial evaluation shows promising feasibility, validity, reliability and ability to discriminate across a range of recruiter practice and evidence of participant understanding. More validation work is needed in new clinical trials to evaluate and refine the measure further.
Keywords: Comprehension; Informed consent; Psychometrics; Randomised controlled trials; Recruitment.
Figures
References
-
- World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Adopted by the 18th World Medical Assembly, Helsinki, Finland, June 1964 and amended 2013. Available at https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-pr.... Accessed 7 Jan 2016.
-
- Council for International Organizations of Medical Sciences (CIOMS) International Ethical Guidelines for Biomedical Research Involving Human Subjects. 2002, Geneva, Switzerland. 2002. - PubMed
-
- US Department of Health and Human Services. Code of Federal Regulations, 21 Part 50 Protection of Human Subjects and 45 part 46 Protection of Human Subjects, Federal Register 18 June 1991;56:28012. Available at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?C.... Accessed 7 Jan 2016.
-
- Beauchamp TL, Childress JF. Principles of biomedical ethics. 4. Oxford: Oxford University Press; 1994.
-
- US Food and Drug Administration, Department of Health and Human Services . Draft guidance: informed consent information sheet. Guidance for IRBs, clinical investigators, and sponsors. Silver Spring, MD: US Food and Drug Administration, Department of Health and Human Services; 2014.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources