A nested mechanistic sub-study into the effect of tranexamic acid versus placebo on intracranial haemorrhage and cerebral ischaemia in isolated traumatic brain injury: study protocol for a randomised controlled trial (CRASH-3 Trial Intracranial Bleeding Mechanistic Sub-Study [CRASH-3 IBMS])
- PMID: 28716153
- PMCID: PMC5513059
- DOI: 10.1186/s13063-017-2073-6
A nested mechanistic sub-study into the effect of tranexamic acid versus placebo on intracranial haemorrhage and cerebral ischaemia in isolated traumatic brain injury: study protocol for a randomised controlled trial (CRASH-3 Trial Intracranial Bleeding Mechanistic Sub-Study [CRASH-3 IBMS])
Abstract
Background: Tranexamic acid prevents blood clots from breaking down and reduces bleeding. However, it is uncertain whether tranexamic acid is effective in traumatic brain injury. The CRASH-3 trial is a randomised controlled trial that will examine the effect of tranexamic acid (versus placebo) on death and disability in 13,000 patients with traumatic brain injury. The CRASH-3 trial hypothesizes that tranexamic acid will reduce intracranial haemorrhage, which will reduce the risk of death. Although it is possible that tranexamic acid will reduce intracranial bleeding, there is also a potential for harm. In particular, tranexamic acid may increase the risk of cerebral thrombosis and ischaemia. The protocol detailed here is for a mechanistic sub-study nested within the CRASH-3 trial. This mechanistic sub-study aims to examine the effect of tranexamic acid (versus placebo) on intracranial bleeding and cerebral ischaemia.
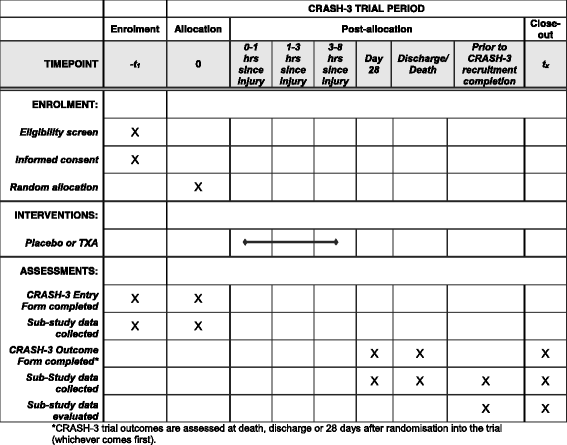
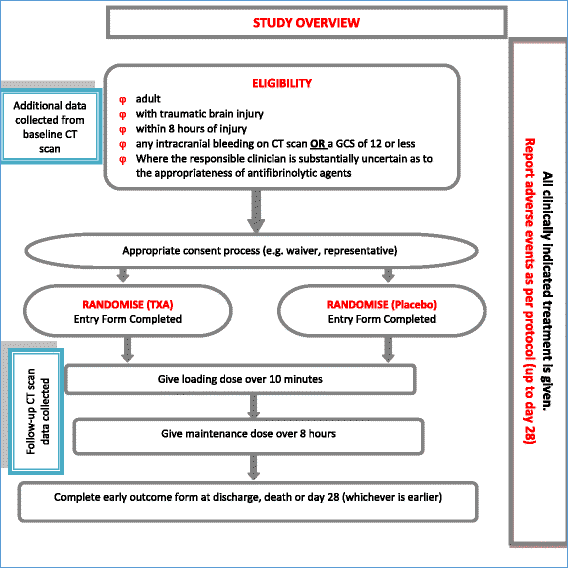
Methods: The CRASH-3 Intracranial Bleeding Mechanistic Sub-Study (CRASH-3 IBMS) is nested within a prospective, double-blind, multi-centre, parallel-arm randomised trial called the CRASH-3 trial. The CRASH-3 IBMS will be conducted in a cohort of approximately 1000 isolated traumatic brain injury patients enrolled in the CRASH-3 trial. In the CRASH-3 IBMS, brain scans acquired before and after randomisation are examined, using validated methods, for evidence of intracranial bleeding and cerebral ischaemia. The primary outcome is the total volume of intracranial bleeding measured on computed tomography after randomisation, adjusting for baseline bleeding volume. Secondary outcomes include progression of intracranial haemorrhage (from pre- to post-randomisation scans), new intracranial haemorrhage (seen on post- but not pre-randomisation scans), intracranial haemorrhage following neurosurgery, and new focal ischaemic lesions (seen on post-but not pre-randomisation scans). A linear regression model will examine whether receipt of the trial treatment can predict haemorrhage volume. Bleeding volumes and new ischaemic lesions will be compared across treatment groups using relative risks and 95% confidence intervals.
Discussion: The CRASH-3 IBMS will provide an insight into the mechanism of action of tranexamic acid in traumatic brain injury, as well as information about the risks and benefits. Evidence from this trial could inform the management of patients with traumatic brain injury.
Trial registration: The CRASH-3 trial was prospectively registered and the CRASH-3 IBMS is an addition to the original protocol registered at the International Standard Randomised Controlled Trials registry ( ISRCTN15088122 ) 19 July 2011, and ClinicalTrials.gov on 25 July 2011 (NCT01402882).
Keywords: Cerebral ischaemia; Intracranial haemorrhage; Tranexamic acid; Traumatic brain injury.
Conflict of interest statement
Authors’ information
All authors are from the Clinical Trials Unit, Faculty of Epidemiology and Population Health at the London School of Hygiene and Tropical Medicine (University of London).
Ethics approval and consent to participate
The Medical Research and Ethics Committee and Health Research Authority reviewed the protocol and supporting documents for the CRASH-3 IBMS and provided a favourable ethical opinion on 8 June 2016 (Research Ethics Committee Reference 12/EE/0274; Additional file 8). These committees approved the CRASH-3 IBMS to be conducted at the Royal London Hospital (London), Queen Elizabeth Hospital (Birmingham), University Hospital (Coventry) and Salford Royal Hospital (Salford), and in additional sites yet to be confirmed. The Royal London Hospital (London), Queen Elizabeth Hospital (Birmingham), University Hospital (Coventry) and Salford Royal Hospital (Salford) have provided local approvals and letters of access for the CRASH-3 IBMS to be conducted at their respective sites. All relevant local ethical approvals will be gained from additional sites.
Favourable ethical opinion was received from the Observational/Interventions Research Ethics Committee at the London School of Hygiene and Tropical Medicine on 24 May 2016 (Reference 11535; Additional file 9). Important protocol modifications will be submitted to and reviewed by the Medical Research and Ethics Committee and Health Research Authority, and registries updated as appropriate.
TBI patients are physically and mentally incapable of providing informed consent to participate in a clinical trial. As acknowledged in the Declaration of Helsinki, patients who are incapable of giving consent are an exception to the general rule of informed consent in clinical trials [77]. In the CRASH-3 trial, patients are unable to provide consent and so consent is sought from the patient’s relative, legal representative or the responsible clinician. If and when the patient regains capacity to provide informed consent, they are informed about the trial and written consent sought to continue their participation in the trial. If a patient or patient representative declines consent, they are withdrawn from the trial. For patients who were included in the trial but did not regain capacity, written informed consent is sought from a relative or legal representative. The requirements of relevant local and national ethics committees are adhered to at all times.
The CRASH-3 trial includes consent to extract data from patient medical records. Collecting CT scan data for the CRASH-3 IBMS is consistent with the consent procedure used in the CRASH-3 trial. It would be impractical to re-consent patients or relatives/legal representatives to access CT scans, particularly for patients who have deceased or are disabled as a result of their injuries where re-consent would be distressing and unwelcome. The London School of Hygiene and Tropical Medicine and national Ethics Committees extended their approvals to extract CT data from the CRASH-3 trial without further patient consent. Patients who withdrew from the main CRASH-3 trial would not be included in the CRASH-3 IBMS.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007;22(5):341–53. - PubMed
-
- Hancock CBHJ. The global burden of traumatic brain injury: preliminary results from the Global Burden of Disease Project. Inj Prev. 2010;16:A17. doi: 10.1136/ip.2010.029215.61. - DOI
-
- Bunch J, Jennifer H. Information and Resources Column. TBI challenge 4, No. 2. Alexandria: BIA; 2000.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical