Microglia control the glycinergic but not the GABAergic synapses via prostaglandin E2 in the spinal cord
- PMID: 28716844
- PMCID: PMC5584146
- DOI: 10.1083/jcb.201607048
Microglia control the glycinergic but not the GABAergic synapses via prostaglandin E2 in the spinal cord
Abstract
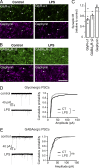
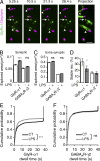
Microglia control excitatory synapses, but their role in inhibitory neurotransmission has been less well characterized. Herein, we show that microglia control the strength of glycinergic but not GABAergic synapses via modulation of the diffusion dynamics and synaptic trapping of glycine (GlyR) but not GABAA receptors. We further demonstrate that microglia regulate the activity-dependent plasticity of glycinergic synapses by tuning the GlyR diffusion trap. This microglia-synapse cross talk requires production of prostaglandin E2 by microglia, leading to the activation of neuronal EP2 receptors and cyclic adenosine monophosphate-dependent protein kinase. Thus, we now provide a link between microglial activation and synaptic dysfunctions, which are common early features of many brain diseases.
© 2017 Cantaut-Belarif et al.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

