Protein kinase D at the Golgi controls NLRP3 inflammasome activation
- PMID: 28716882
- PMCID: PMC5584123
- DOI: 10.1084/jem.20162040
Protein kinase D at the Golgi controls NLRP3 inflammasome activation
Abstract
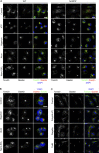
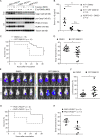
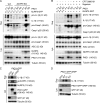
The inflammasomes are multiprotein complexes sensing tissue damage and infectious agents to initiate innate immune responses. Different inflammasomes containing distinct sensor molecules exist. The NLRP3 inflammasome is unique as it detects a variety of danger signals. It has been reported that NLRP3 is recruited to mitochondria-associated endoplasmic reticulum membranes (MAMs) and is activated by MAM-derived effectors. Here, we show that in response to inflammasome activators, MAMs localize adjacent to Golgi membranes. Diacylglycerol (DAG) at the Golgi rapidly increases, recruiting protein kinase D (PKD), a key effector of DAG. Upon PKD inactivation, self-oligomerized NLRP3 is retained at MAMs adjacent to Golgi, blocking assembly of the active inflammasome. Importantly, phosphorylation of NLRP3 by PKD at the Golgi is sufficient to release NLRP3 from MAMs, resulting in assembly of the active inflammasome. Moreover, PKD inhibition prevents inflammasome autoactivation in peripheral blood mononuclear cells from patients carrying NLRP3 mutations. Hence, Golgi-mediated PKD signaling is required and sufficient for NLRP3 inflammasome activation.
© 2017 Zhang et al.
Figures







References
-
- Abe K., Fuchs H., Boersma A., Hans W., Yu P., Kalaydjiev S., Klaften M., Adler T., Calzada-Wack J., Mossbrugger I., et al. . 2011. A novel N-ethyl-N-nitrosourea-induced mutation in phospholipase Cγ2 causes inflammatory arthritis, metabolic defects, and male infertility in vitro in a murine model. Arthritis Rheum. 63:1301–1311. 10.1002/art.30280 - DOI - PubMed
-
- Aksentijevich I., Putnam C.D., Remmers E.F., Mueller J.L., Le J., Kolodner R.D., Moak Z., Chuang M., Austin F., Goldbach-Mansky R., et al. . 2007. The clinical continuum of cryopyrinopathies: Novel CIAS1 mutations in North American patients and a new cryopyrin model. Arthritis Rheum. 56:1273–1285. 10.1002/art.22491 - DOI - PMC - PubMed
-
- Baroja-Mazo A., Martín-Sánchez F., Gomez A.I., Martínez C.M., Amores-Iniesta J., Compan V., Barberà-Cremades M., Yagüe J., Ruiz-Ortiz E., Antón J., et al. . 2014. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat. Immunol. 15:738–748. 10.1038/ni.2919 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

