CTLA4 Promotes Tyk2-STAT3-Dependent B-cell Oncogenicity
- PMID: 28716895
- PMCID: PMC5600851
- DOI: 10.1158/0008-5472.CAN-16-0342
CTLA4 Promotes Tyk2-STAT3-Dependent B-cell Oncogenicity
Abstract
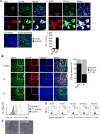
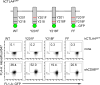
CTL-associated antigen 4 (CTLA4) is a well-established immune checkpoint for antitumor immune responses. The protumorigenic function of CTLA4 is believed to be limited to T-cell inhibition by countering the activity of the T-cell costimulating receptor CD28. However, as we demonstrate here, there are two additional roles for CTLA4 in cancer, including via CTLA4 overexpression in diverse B-cell lymphomas and in melanoma-associated B cells. CTLA4-CD86 ligation recruited and activated the JAK family member Tyk2, resulting in STAT3 activation and expression of genes critical for cancer immunosuppression and tumor growth and survival. CTLA4 activation resulted in lymphoma cell proliferation and tumor growth, whereas silencing or antibody-blockade of CTLA4 in B-cell lymphoma tumor cells in the absence of T cells inhibits tumor growth. This inhibition was accompanied by reduction of Tyk2/STAT3 activity, tumor cell proliferation, and induction of tumor cell apoptosis. The CTLA4-Tyk2-STAT3 signal pathway was also active in tumor-associated nonmalignant B cells in mouse models of melanoma and lymphoma. Overall, our results show how CTLA4-induced immune suppression occurs primarily via an intrinsic STAT3 pathway and that CTLA4 is critical for B-cell lymphoma proliferation and survival. Cancer Res; 77(18); 5118-28. ©2017 AACR.
©2017 American Association for Cancer Research.
Figures




References
-
- Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18:206–13. - PubMed
-
- Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–50. - PubMed
-
- Mantovani A. B cells and macrophages in cancer: yin and yang. Nat Med. 2011;17:285–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

