MitoNEET-dependent formation of intermitochondrial junctions
- PMID: 28716905
- PMCID: PMC5547643
- DOI: 10.1073/pnas.1706643114
MitoNEET-dependent formation of intermitochondrial junctions
Abstract
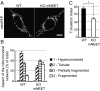
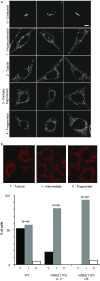
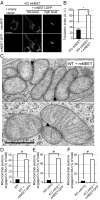
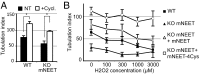
MitoNEET (mNEET) is a dimeric mitochondrial outer membrane protein implicated in many facets of human pathophysiology, notably diabetes and cancer, but its molecular function remains poorly characterized. In this study, we generated and analyzed mNEET KO cells and found that in these cells the mitochondrial network was disturbed. Analysis of 3D-EM reconstructions and of thin sections revealed that genetic inactivation of mNEET did not affect the size of mitochondria but that the frequency of intermitochondrial junctions was reduced. Loss of mNEET decreased cellular respiration, because of a reduction in the total cellular mitochondrial volume, suggesting that intermitochondrial contacts stabilize individual mitochondria. Reexpression of mNEET in mNEET KO cells restored the WT morphology of the mitochondrial network, and reexpression of a mutant mNEET resistant to oxidative stress increased in addition the resistance of the mitochondrial network to H2O2-induced fragmentation. Finally, overexpression of mNEET increased strongly intermitochondrial contacts and resulted in the clustering of mitochondria. Our results suggest that mNEET plays a specific role in the formation of intermitochondrial junctions and thus participates in the adaptation of cells to physiological changes and to the control of mitochondrial homeostasis.
Keywords: CISD1; endoplasmic reticulum; intermitochondrial junctions; mitoNEET; mitochondria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Giampazolias E, Tait SW. Mitochondria and the hallmarks of cancer. FEBS J. 2016;283:803–814. - PubMed
-
- Archer SL. Mitochondrial dynamics–Mitochondrial fission and fusion in human diseases. N Engl J Med. 2013;369:2236–2251. - PubMed
-
- Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–780. - PubMed
-
- Colca JR, et al. Identification of a novel mitochondrial protein (“mitoNEET”) cross-linked specifically by a thiazolidinedione photoprobe. Am J Physiol Endocrinol Metab. 2004;286:E252–E260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

