Sialylation on O-glycans protects platelets from clearance by liver Kupffer cells
- PMID: 28716912
- PMCID: PMC5547648
- DOI: 10.1073/pnas.1707662114
Sialylation on O-glycans protects platelets from clearance by liver Kupffer cells
Abstract
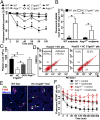
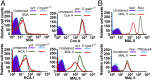
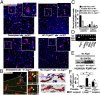
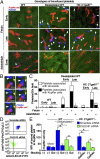
Most platelet membrane proteins are modified by mucin-type core 1-derived glycans (O-glycans). However, the biological importance of O-glycans in platelet clearance is unclear. Here, we generated mice with a hematopoietic cell-specific loss of O-glycans (HC C1galt1-/- ). These mice lack O-glycans on platelets and exhibit reduced peripheral platelet numbers. Platelets from HC C1galt1-/- mice show reduced levels of α-2,3-linked sialic acids and increased accumulation in the liver relative to wild-type platelets. The preferential accumulation of HC C1galt1-/- platelets in the liver was reduced in mice lacking the hepatic asialoglycoprotein receptor [Ashwell-Morell receptor (AMR)]. However, we found that Kupffer cells are the primary cells phagocytosing HC C1galt1-/- platelets in the liver. Our results demonstrate that hepatic AMR promotes preferential adherence to and phagocytosis of desialylated and/or HC C1galt1-/- platelets by the Kupffer cell through its C-type lectin receptor CLEC4F. These findings provide insights into an essential role for core 1 O-glycosylation of platelets in their clearance in the liver.
Keywords: Kupffer cell; O-glycan; clearance; platelet.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

