Metabolic profiles of exercise in patients with McArdle disease or mitochondrial myopathy
- PMID: 28716914
- PMCID: PMC5547614
- DOI: 10.1073/pnas.1703338114
Metabolic profiles of exercise in patients with McArdle disease or mitochondrial myopathy
Abstract
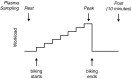
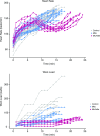
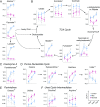
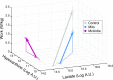
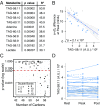
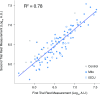
McArdle disease and mitochondrial myopathy impair muscle oxidative phosphorylation (OXPHOS) by distinct mechanisms: the former by restricting oxidative substrate availability caused by blocked glycogen breakdown, the latter because of intrinsic respiratory chain defects. We applied metabolic profiling to systematically interrogate these disorders at rest, when muscle symptoms are typically minimal, and with exercise, when symptoms of premature fatigue and potential muscle injury are unmasked. At rest, patients with mitochondrial disease exhibit elevated lactate and reduced uridine; in McArdle disease purine nucleotide metabolites, including xanthine, hypoxanthine, and inosine are elevated. During exercise, glycolytic intermediates, TCA cycle intermediates, and pantothenate expand dramatically in both mitochondrial disease and control subjects. In contrast, in McArdle disease, these metabolites remain unchanged from rest; but urea cycle intermediates are increased, likely attributable to increased ammonia production as a result of exaggerated purine degradation. Our results establish skeletal muscle glycogen as the source of TCA cycle expansion that normally accompanies exercise and imply that impaired TCA cycle flux is a central mechanism of restricted oxidative capacity in this disorder. Finally, we report that resting levels of long-chain triacylglycerols in mitochondrial myopathy correlate with the severity of OXPHOS dysfunction, as indicated by the level of impaired O2 extraction from arterial blood during peak exercise. Our integrated analysis of exercise and metabolism provides unique insights into the biochemical basis of these muscle oxidative defects, with potential implications for their clinical management.
Keywords: McArdle disease; TCA expansion; exercise physiology; metabolic profiling; mitochondria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Chen Y-T, Kishnani PS, Koeberl D. 2014. Glycogen storage diseases. OMMBID: The Online Metabolic and Molecular Bases of Inherited Diseases, eds Valle D, et al. (McGraw Hill, New York), Chap 71.
-
- McArdle B. Myopathy due to a defect in muscle glycogen breakdown. Clin Sci. 1951;10:13–35. - PubMed
-
- Rumpf KW, et al. Increased ammonia production during forearm ischemic work test in McArdle’s disease. Klin Wochenschr. 1981;59:1319–1320. - PubMed
-
- Haller RG, Vissing J. Spontaneous “second wind” and glucose-induced second “second wind” in McArdle disease: Oxidative mechanisms. Arch Neurol. 2002;59:1395–1402. - PubMed
-
- Taivassalo T, et al. The spectrum of exercise tolerance in mitochondrial myopathies: A study of 40 patients. Brain. 2003;126:413–423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

