3D printed scaffolds of calcium silicate-doped β-TCP synergize with co-cultured endothelial and stromal cells to promote vascularization and bone formation
- PMID: 28717129
- PMCID: PMC5514115
- DOI: 10.1038/s41598-017-05196-1
3D printed scaffolds of calcium silicate-doped β-TCP synergize with co-cultured endothelial and stromal cells to promote vascularization and bone formation
Abstract
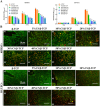
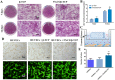
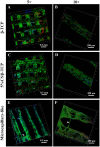
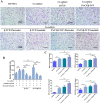
Synthetic bone scaffolds have potential application in repairing large bone defects, however, inefficient vascularization after implantation remains the major issue of graft failure. Herein, porous β-tricalcium phosphate (β-TCP) scaffolds with calcium silicate (CS) were 3D printed, and pre-seeded with co-cultured human umbilical cord vein endothelial cells (HUVECs) and human bone marrow stromal cells (hBMSCs) to construct tissue engineering scaffolds with accelerated vascularization and better bone formation. Results showed that in vitro β-TCP scaffolds doped with 5% CS (5%CS/β-TCP) were biocompatible, and stimulated angiogenesis and osteogenesis. The results also showed that 5%CS/β-TCP scaffolds not only stimulated co-cultured cells angiogenesis on Matrigel, but also stimulated co-cultured cells to form microcapillary-like structures on scaffolds, and promoted migration of BMSCs by stimulating co-cultured cells to secrete PDGF-BB and CXCL12 into the surrounding environment. Moreover, 5%CS/β-TCP scaffolds enhanced vascularization and osteoinduction in comparison with β-TCP, and synergized with co-cultured cells to further increase early vessel formation, which was accompanied by earlier and better ectopic bone formation when implanted subcutaneously in nude mice. Thus, our findings suggest that porous 5%CS/β-TCP scaffolds seeded with co-cultured cells provide new strategy for accelerating tissue engineering scaffolds vascularization and osteogenesis, and show potential as treatment for large bone defects.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

