Extracellular vesicles released following heat stress induce bystander effect in unstressed populations
- PMID: 28717426
- PMCID: PMC5505002
- DOI: 10.1080/20013078.2017.1340746
Extracellular vesicles released following heat stress induce bystander effect in unstressed populations
Abstract
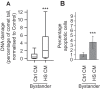
Cells naïve to stress can display the effects of stress, such as DNA damage and apoptosis, when they are exposed to signals from stressed cells; this phenomenon is known as the bystander effect. We previously showed that bystander effect induced by ionising radiation are mediated by extracellular vesicles (EVs). Bystander effect can also be induced by other types of stress, including heat shock, but it is unclear whether EVs are involved. Here we show that EVs released from heat shocked cells are also able to induce bystander damage in unstressed populations. Naïve cells treated with media conditioned by heat shocked cells showed higher levels of DNA damage and apoptosis than cells treated with media from control cells. Treating naïve cells with EVs derived from media conditioned by heat shocked cells also induced a bystander effect when compared to control, with DNA damage and apoptosis increasing whilst the level of cell viability was reduced. We demonstrate that treatment of naïve cells with heat shocked cell-derived EVs leads to greater invasiveness in a trans-well Matrigel assay. Finally, we show that naïve cells treated with EVs from heat-shocked cells are more likely to survive a subsequent heat shock, suggesting that these EVs mediate an adaptive response. We propose that EVs released following stress mediate an intercellular response that leads to apparent stress in neighbouring cells but also greater robustness in the face of a subsequent insult.
Keywords: Cancer; DNA damage; apoptosis; intercellular communication; stress response.
Figures




References
-
- Hall EJ. The bystander effect. Health Phys. 2003;85(1):31–10. - PubMed
-
- Hickman AW, Jaramillo RJ, Lechner JF, et al. Alpha-particle-induced p53 protein expression in a rat lung epithelial cell strain. Cancer Res. 1994;54(22):5797–5800. - PubMed
-
- Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of α -particles. Cancer Res. 1992;2115(4):6394–6396. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

