Boron chemistry in a new light
- PMID: 28717443
- PMCID: PMC5502392
- DOI: 10.1039/c5sc02207j
Boron chemistry in a new light
Abstract
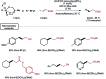
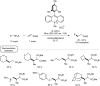
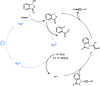
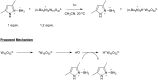
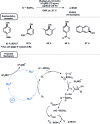
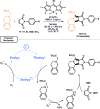
Photocatalysis has recently opened up new avenues for the generation of radical species under visible light irradiation conditions. A particularly fascinating class of photocatalyzed transformations relies on the activation of stable boron species with visible-light since it allows the creation of boryl and/or carbon radicals through single electron transfer or energy transfer without the need for specific and costly equipment. This new paradigm has found numerous applications in synthetic organic chemistry, catalysis, and macromolecular chemistry. In this minireview, the concepts underlying photoactivation of boron-species as well as applications to the creation of C-H, C-C, C-O, B-C and B-S bond are discussed.
Figures


























References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

