Fusion to Flaviviral Leader Peptide Targets HIV-1 Reverse Transcriptase for Secretion and Reduces Its Enzymatic Activity and Ability to Induce Oxidative Stress but Has No Major Effects on Its Immunogenic Performance in DNA-Immunized Mice
- PMID: 28717654
- PMCID: PMC5498913
- DOI: 10.1155/2017/7407136
Fusion to Flaviviral Leader Peptide Targets HIV-1 Reverse Transcriptase for Secretion and Reduces Its Enzymatic Activity and Ability to Induce Oxidative Stress but Has No Major Effects on Its Immunogenic Performance in DNA-Immunized Mice
Abstract
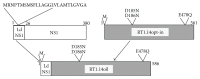
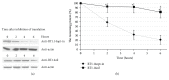
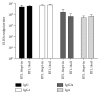
Reverse transcriptase (RT) is a key enzyme in viral replication and susceptibility to ART and a crucial target of immunotherapy against drug-resistant HIV-1. RT induces oxidative stress which undermines the attempts to make it immunogenic. We hypothesized that artificial secretion may reduce the stress and make RT more immunogenic. Inactivated multidrug-resistant RT (RT1.14opt-in) was N-terminally fused to the signal providing secretion of NS1 protein of TBEV (Ld) generating optimized inactivated Ld-carrying enzyme RT1.14oil. Promotion of secretion prohibited proteasomal degradation increasing the half-life and content of RT1.14oil in cells and cell culture medium, drastically reduced the residual polymerase activity, and downmodulated oxidative stress. BALB/c mice were DNA-immunized with RT1.14opt-in or parental RT1.14oil by intradermal injections with electroporation. Fluorospot and ELISA tests revealed that RT1.14opt-in and RT1.14oil induced IFN-γ/IL-2, RT1.14opt-in induced granzyme B, and RT1.14oil induced perforin production. Perforin secretion correlated with coproduction of IFN-γ and IL-2 (R = 0,97). Both DNA immunogens induced strong anti-RT antibody response. Ld peptide was not immunogenic. Thus, Ld-driven secretion inferred little change to RT performance in DNA immunization. Positive outcome was the abrogation of polymerase activity increasing safety of RT-based DNA vaccines. Identification of the molecular determinants of low cellular immunogenicity of RT requires further studies.
Figures






Similar articles
-
Codon optimization and improved delivery/immunization regimen enhance the immune response against wild-type and drug-resistant HIV-1 reverse transcriptase, preserving its Th2-polarity.Sci Rep. 2018 May 24;8(1):8078. doi: 10.1038/s41598-018-26281-z. Sci Rep. 2018. PMID: 29799015 Free PMC article.
-
Potent cross-reactive immune response against the wild-type and drug-resistant forms of HIV reverse transcriptase after the chimeric gene immunization.Vaccine. 2010 Feb 23;28(8):1975-86. doi: 10.1016/j.vaccine.2009.10.098. Vaccine. 2010. PMID: 20188253
-
Oxidative stress induced by HIV-1 reverse transcriptase modulates the enzyme's performance in gene immunization.Hum Vaccin Immunother. 2013 Oct;9(10):2111-9. doi: 10.4161/hv.25813. Epub 2013 Jul 23. Hum Vaccin Immunother. 2013. PMID: 23881028 Free PMC article.
-
HIV-1 reverse transcriptase artificially targeted for proteasomal degradation induces a mixed Th1/Th2-type immune response.Vaccine. 2008 Sep 19;26(40):5170-6. doi: 10.1016/j.vaccine.2008.03.070. Epub 2008 Apr 15. Vaccine. 2008. PMID: 18468738
-
A truncated plasmid-encoded HIV-1 reverse transcriptase displays strong immunogenicity.Viral Immunol. 2013 Apr;26(2):163-6. doi: 10.1089/vim.2012.0083. Viral Immunol. 2013. PMID: 23573980
Cited by
-
Oncogenic Effects of HIV-1 Proteins, Mechanisms Behind.Cancers (Basel). 2021 Jan 15;13(2):305. doi: 10.3390/cancers13020305. Cancers (Basel). 2021. PMID: 33467638 Free PMC article. Review.
-
HIV-1 Reverse Transcriptase Promotes Tumor Growth and Metastasis Formation via ROS-Dependent Upregulation of Twist.Oxid Med Cell Longev. 2019 Dec 2;2019:6016278. doi: 10.1155/2019/6016278. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31885806 Free PMC article.
-
The Immunogenicity in Mice of HCV Core Delivered as DNA Is Modulated by Its Capacity to Induce Oxidative Stress and Oxidative Stress Response.Cells. 2019 Feb 28;8(3):208. doi: 10.3390/cells8030208. Cells. 2019. PMID: 30823485 Free PMC article.
-
Codon optimization and improved delivery/immunization regimen enhance the immune response against wild-type and drug-resistant HIV-1 reverse transcriptase, preserving its Th2-polarity.Sci Rep. 2018 May 24;8(1):8078. doi: 10.1038/s41598-018-26281-z. Sci Rep. 2018. PMID: 29799015 Free PMC article.
-
Expression of the Reverse Transcriptase Domain of Telomerase Reverse Transcriptase Induces Lytic Cellular Response in DNA-Immunized Mice and Limits Tumorigenic and Metastatic Potential of Murine Adenocarcinoma 4T1 Cells.Vaccines (Basel). 2020 Jun 18;8(2):318. doi: 10.3390/vaccines8020318. Vaccines (Basel). 2020. PMID: 32570805 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

