The effects of high concentrations of ionic liquid on GB1 protein structure and dynamics probed by high-resolution magic-angle-spinning NMR spectroscopy
- PMID: 28717785
- PMCID: PMC5510950
- DOI: 10.1016/j.bbrep.2016.08.009
The effects of high concentrations of ionic liquid on GB1 protein structure and dynamics probed by high-resolution magic-angle-spinning NMR spectroscopy
Abstract
Ionic liquids have great potential in biological applications and biocatalysis, as some ionic liquids can stabilize proteins and enhance enzyme activity, while others have the opposite effect. However, on the molecular level, probing ionic liquid interactions with proteins, especially in solutions containing high concentration of ionic liquids, has been challenging. In the present work the 13C, 15N-enriched GB1 model protein was used to demonstrate applicability of high-resolution magic-angle-spinning (HR-MAS) NMR spectroscopy to investigate ionic liquid - protein interactions. Effect of an ionic liquid (1-butyl-3-methylimidazolium bromide, [C4-mim]Br) on GB1was studied over a wide range of the ionic liquid concentrations (0.6 to 3.5 M, which corresponds to 10%-60% v/v). Interactions between GB1 and [C4-mim]Br were observed from changes in the chemical shifts of the protein backbone as well as the changes in 15N ps-ns dynamics and rotational correlation times. Site-specific interactions between the protein and [C4-mim]Br were assigned using 3D methods under HR-MAS conditions. Thus, HR-MAS NMR is a viable tool that could aid in elucidation of the molecular mechanism of ionic liquid - protein interactions.
Keywords: GB1; HR-MAS NMR; imidazolium ionic liquid; ionic liquid – protein interaction.
Figures
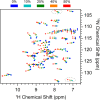


References
-
- Hallett J.P., Welton T. Room-temperature ionic liquids: solvents for synthesis and catalysis. 2. Chem. Rev. 2011;111:3508–3576. - PubMed
-
- van Rantwijk F., Sheldon R.A. Biocatalysis in ionic liquids. Chem. Rev. 2007;107:2757–2785. - PubMed
-
- Santos E., Albo J., Irabien A. Magnetic ionic liquids: synthesis, properties and applications. RSC Adv. 2014;4:40008–40018.
-
- Neouze M.A., Kronstein M., Tielens F. Ionic nanoparticle networks: development and perspectives in the landscape of ionic liquid based materials. Chem. Commun. 2014;50:10929–10936. - PubMed
-
- Ma M.G., Zhu J.F., Zhu Y.J., Sun R.C. The microwave-assisted ionic-liquid method: a promising methodology in nanomaterials. Chem.: Asian J. 2014;9:2378–2391. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
