Structural and Ultrastructural Changes to Type I Spiral Ganglion Neurons and Schwann Cells in the Deafened Guinea Pig Cochlea
- PMID: 28717876
- PMCID: PMC5688041
- DOI: 10.1007/s10162-017-0631-y
Structural and Ultrastructural Changes to Type I Spiral Ganglion Neurons and Schwann Cells in the Deafened Guinea Pig Cochlea
Abstract
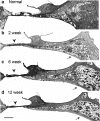
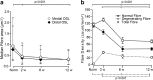
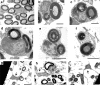
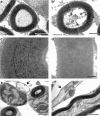
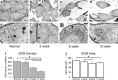
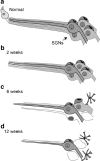
Sensorineural hearing loss is commonly caused by damage to cochlear sensory hair cells. Coinciding with hair cell degeneration, the peripheral fibres of type I spiral ganglion neurons (SGNs) that normally form synaptic connections with the inner hair cell gradually degenerate. We examined the time course of these degenerative changes in type I SGNs and their satellite Schwann cells at the ultrastructural level in guinea pigs at 2, 6, and 12 weeks following aminoglycoside-induced hearing loss. Degeneration of the peripheral fibres occurred prior to the degeneration of the type I SGN soma and was characterised by shrinkage of the fibre followed by retraction of the axoplasm, often leaving a normal myelin lumen devoid of axoplasmic content. A statistically significant reduction in the cross-sectional area of peripheral fibres was evident as early as 2 weeks following deafening (p < 0.001, ANOVA). This was followed by a decrease in type I SGN density within Rosenthal's canal that was statistically significant 6 weeks following deafening (p < 0.001, ANOVA). At any time point examined, few type I SGN soma were observed undergoing degeneration, implying that once initiated, soma degeneration was rapid. While there was a significant reduction in soma area as well as changes to the morphology of the soma, the ultrastructure of surviving type I SGN soma appeared relatively normal over the 12-week period following deafening. Satellite Schwann cells exhibited greater survival traits than their type I SGN; however, on loss of neural contact, they reverted to a non-myelinating phenotype, exhibiting an astrocyte-like morphology with the formation of processes that appeared to be searching for new neural targets. In 6- and 12-week deafened cochlea, we observed cellular interaction between Schwann cell processes and residual SGNs that distorted the morphology of the SGN soma. Understanding the response of SGNs, Schwann cells, and the complex relationship between them following aminoglycoside deafening is important if we are to develop effective therapeutic techniques designed to rescue SGNs.
Keywords: Schwann cell; deafness; nerve degeneration, nerve regeneration, cochlear implant; spiral ganglion neuron.
Conflict of interest statement
Conflict of Interest
The authors declare that they have no conflict of interest.
Role of Authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: AKW, RP, TGL, JBF, and RKS. Acquisition of data: AKW, RP, and TGL. Analysis and interpretation of data: AKW, RP, TGL, JBF, and RKS. Drafting of the manuscript: AKW, RP, TGL, JBF, and RKS. Critical revision of the manuscript for important intellectual content: AKW, RP, TGL, JBF, and RKS. Statistical analysis: AKW and TGL. Obtained funding: RKS and AKW. Study supervision: AKW.
Figures






References
-
- Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

