Hypertrophic cardiomyopathy and the myosin mesa: viewing an old disease in a new light
- PMID: 28717924
- PMCID: PMC5803174
- DOI: 10.1007/s12551-017-0274-6
Hypertrophic cardiomyopathy and the myosin mesa: viewing an old disease in a new light
Abstract
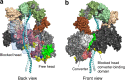
The sarcomere is an exquisitely designed apparatus that is capable of generating force, which in the case of the heart results in the pumping of blood throughout the body. At the molecular level, an ATP-dependent interaction of myosin with actin drives the contraction and force generation of the sarcomere. Over the past six decades, work on muscle has yielded tremendous insights into the workings of the sarcomeric system. We now stand on the cusp where the acquired knowledge of how the sarcomere contracts and how that contraction is regulated can be extended to an understanding of the molecular mechanisms of sarcomeric diseases, such as hypertrophic cardiomyopathy (HCM). In this review we present a picture that combines current knowledge of the myosin mesa, the sequestered state of myosin heads on the thick filament, known as the interacting-heads motif (IHM), their possible interaction with myosin binding protein C (MyBP-C) and how these interactions can be abrogated leading to hyper-contractility, a key clinical manifestation of HCM. We discuss the structural and functional basis of the IHM state of the myosin heads and identify HCM-causing mutations that can directly impact the equilibrium between the 'on state' of the myosin heads (the open state) and the IHM 'off state'. We also hypothesize a role of MyBP-C in helping to maintain myosin heads in the IHM state on the thick filament, allowing release in a graded manner upon adrenergic stimulation. By viewing clinical hyper-contractility as the result of the destabilization of the IHM state, our aim is to view an old disease in a new light.
Keywords: Dilated cardiomyopathy; Hypertrophic cardiomyopathy; Interacting-heads motif; Myosin binding protein C; Myosin mesa; Myosin sequestered state.
Conflict of interest statement
Funding
This work was funded by NIH grants GM33289 and HL117138 to J.A.S., a Stanford Lucile Packard CHRI Postdoctoral Award (UL1 TR001085) and American Heart Association Postdoctoral Fellowship (17POST33411070) to D.V.T., and a Stanford Lucile Packard CHRI Postdoctoral Award (UL1 TR001085), Stanford ChEM-H Postdocs at the Interface Award, and American Heart Association Postdoctoral Fellowship (16POST30890005) to ASA.
Conflict of interest
J.A.S. is a founder of Cytokinetics and MyoKardia and a member of their scientific advisory boards. K.M.R. is on the SAB of MyoKardia.
Ethical approval
This manuscript does not contain any work involving human subjects or animal models.
Figures




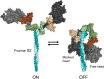

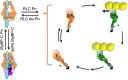
References
-
- Adhikari AS, Kooiker KB, Sarkar SS, Liu C, Bernstein D, Spudich JA, Ruppel KM. Early-onset hypertrophic cardiomyopathy mutations significantly increase the velocity, force, and actin-activated ATPase activity of human beta-cardiac myosin. Cell Rep. 2016;17:2857–2864. doi: 10.1016/j.celrep.2016.11.040. - DOI - PMC - PubMed
-
- Ait-Mou Y, Hsu K, Farman GP, Kumar M, Greaser ML, Irving TC, de Tombe PP. Titin strain contributes to the frank-Starling law of the heart by structural rearrangements of both thin- and thick-filament proteins. Proc Natl Acad Sci USA. 2016;113:2306–2311. doi: 10.1073/pnas.1516732113. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

