A pyrrole-based natural small molecule mitigates HSP90 expression in MDA-MB-231 cells and inhibits tumor angiogenesis in mice by inactivating HSF-1
- PMID: 28717943
- PMCID: PMC5573693
- DOI: 10.1007/s12192-017-0802-0
A pyrrole-based natural small molecule mitigates HSP90 expression in MDA-MB-231 cells and inhibits tumor angiogenesis in mice by inactivating HSF-1
Abstract
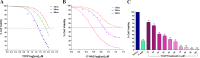
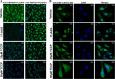
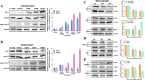
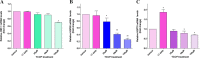
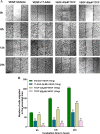
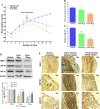
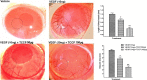
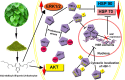
Heat shock proteins (HSPs), molecular chaperones, are crucial for the cancer cells to facilitate proper functioning of various oncoproteins involved in cell survival, proliferation, migration, and tumor angiogenesis. Tumor cells are said to be "addicted" to HSPs. HSPs are overexpressed in many cancers due to upregulation of transcription factor Heat-shock factor 1 (HSF-1), the multifaceted master regulator of heat shock response. Therefore, pharmacological targeting of HSPs via HSF-1 is an effective strategy to treat malignant cancers like triple negative breast cancer. In the current study, we evaluated the efficacy of a pyrrole derivative [bis(2-ethylhexyl)1H-pyrrole-3,4-dicarboxylate], TCCP, purified from leaves of Tinospora cordifolia for its ability to suppress heat shock response and angiogenesis using MDA-MB-231 cells and the murine mammary carcinoma: Ehrlich ascites tumor model. HSP90 was downregulated by TCCP by inactivation of HSF-1 resulting in inhibition of tumor cell proliferation, VEGF-induced cell migration, and concomitant decrease in tumor burden and neo-angiogenesis in vivo. The mechanism of suppression of HSPs involves inactivation of PI3K/Akt and phosphorylation on serine 307 of HSF-1 by the activation of ERK1. HSF-1 and HSP90 and 70 localization and expression were ascertained by immunolocalization, immunoblotting, and qPCR experiments. The anti-angiogenic effect of TCCP was studied in vivo in tumor-bearing mice and ex vivo using rat corneal micro-pocket assay. All the results thus corroborate the logic behind inactivating HSF-1 using TCCP as an alternative approach for cancer therapy.
Keywords: HSF-1; HSP; MDA-MB-231; NMR; Pyrrole; Tinospora cordifolia.
Figures


Similar articles
-
A new pyrrole based small molecule from Tinospora cordifolia induces apoptosis in MDA-MB-231 breast cancer cells via ROS mediated mitochondrial damage and restoration of p53 activity.Chem Biol Interact. 2019 Feb 1;299:120-130. doi: 10.1016/j.cbi.2018.12.005. Epub 2018 Dec 10. Chem Biol Interact. 2019. PMID: 30543781
-
Opposing actions of phosphatidylinositol 3-kinase and glycogen synthase kinase-3beta in the regulation of HSF-1 activity.J Neurochem. 2000 Dec;75(6):2401-8. doi: 10.1046/j.1471-4159.2000.0752401.x. J Neurochem. 2000. PMID: 11080191
-
Regulation of heat shock protein 72 kDa and 90 kDa in human breast cancer MDA-MB-231 cells.Mol Cell Biochem. 2000 Jan;204(1-2):169-78. doi: 10.1023/a:1007016822939. Mol Cell Biochem. 2000. PMID: 10718636
-
Heat Shock Proteins Promote Cancer: It's a Protection Racket.Trends Biochem Sci. 2016 Apr;41(4):311-323. doi: 10.1016/j.tibs.2016.01.003. Epub 2016 Feb 11. Trends Biochem Sci. 2016. PMID: 26874923 Free PMC article. Review.
-
Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: an update.Arch Toxicol. 2013 Jan;87(1):19-48. doi: 10.1007/s00204-012-0918-z. Epub 2012 Aug 11. Arch Toxicol. 2013. PMID: 22885793 Free PMC article. Review.
Cited by
-
Differential expression of angiogenesis markers HSP70, HSP90, VEGF and pERK1/2 in both components of dedifferentiated chondrosarcomas.J Bone Oncol. 2021 May 18;29:100370. doi: 10.1016/j.jbo.2021.100370. eCollection 2021 Aug. J Bone Oncol. 2021. PMID: 34094840 Free PMC article.
-
Structural Characterization of Heat Shock Protein 90β and Molecular Interactions with Geldanamycin and Ritonavir: A Computational Study.Int J Mol Sci. 2024 Aug 12;25(16):8782. doi: 10.3390/ijms25168782. Int J Mol Sci. 2024. PMID: 39201468 Free PMC article.
-
Comprehensive Analysis of Necroptosis-Related Genes as Prognostic Factors and Immunological Biomarkers in Breast Cancer.J Pers Med. 2022 Dec 26;13(1):44. doi: 10.3390/jpm13010044. J Pers Med. 2022. PMID: 36675706 Free PMC article.
-
HSPA12A Stimulates p38/ERK-AP-1 Signaling to Promote Angiogenesis and Is Required for Functional Recovery Postmyocardial Infarction.Oxid Med Cell Longev. 2022 Jun 22;2022:2333848. doi: 10.1155/2022/2333848. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35783189 Free PMC article.
-
Exploration of anti-leukemic effect of soft coral-derived 13-acetoxysarcocrassolide: Induction of apoptosis via oxidative stress as a potent inhibitor of heat shock protein 90 and topoisomerase II.Kaohsiung J Med Sci. 2023 Jul;39(7):718-731. doi: 10.1002/kjm2.12678. Epub 2023 Apr 13. Kaohsiung J Med Sci. 2023. PMID: 37052190 Free PMC article.
References
-
- Adhvaryu MR, Reddy N, Parabia MH. Anti-tumor activity of four ayurvedic herbs in dalton lymphoma ascites bearing mice and their short-term in vitro cytotoxicity on DLA-cell-line. African Journal of Traditional, Complementary and Alternative Medicines. 2008;5(4):409–418. doi: 10.4314/ajtcam.v5i4.31297. - DOI - PMC - PubMed
-
- Bhardwaj V, Gumber D, Abbot V, Dhiman S, Sharma P. Pyrrole: a resourceful small molecule in key medicinal hetero-aromatics. RSC Adv. 2015;5(20):15233–15266. doi: 10.1039/C4RA15710A. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

