Epigenetics and Cancer Stem Cells: Unleashing, Hijacking, and Restricting Cellular Plasticity
- PMID: 28718414
- PMCID: PMC5506260
- DOI: 10.1016/j.trecan.2017.04.004
Epigenetics and Cancer Stem Cells: Unleashing, Hijacking, and Restricting Cellular Plasticity
Abstract
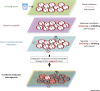
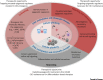
Epigenetic mechanisms have emerged as key players in cancer development which affect cellular states at multiple stages of the disease. During carcinogenesis, alterations in chromatin and DNA methylation resulting from genetic lesions unleash cellular plasticity and favor oncogenic cellular reprogramming. At later stages, during cancer growth and progression, additional epigenetic changes triggered by interaction with the microenvironment modulate cancer cell phenotypes and properties, and shape tumor architecture. We review here recent advances highlighting the interplay between epigenetics, genetics, and cell-to-cell signaling in cancer, with particular emphasis on mechanisms relevant for cancer stem cell formation (CSC) and function.
Keywords: cancer; cancer stem cells; chromatin; epigenetics; intratumoral heterogeneity; plasticity.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

