A connectome of a learning and memory center in the adult Drosophila brain
- PMID: 28718765
- PMCID: PMC5550281
- DOI: 10.7554/eLife.26975
A connectome of a learning and memory center in the adult Drosophila brain
Abstract
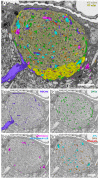
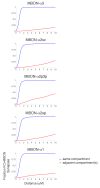
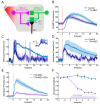
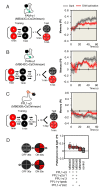
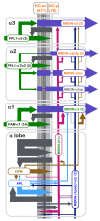
Understanding memory formation, storage and retrieval requires knowledge of the underlying neuronal circuits. In Drosophila, the mushroom body (MB) is the major site of associative learning. We reconstructed the morphologies and synaptic connections of all 983 neurons within the three functional units, or compartments, that compose the adult MB's α lobe, using a dataset of isotropic 8 nm voxels collected by focused ion-beam milling scanning electron microscopy. We found that Kenyon cells (KCs), whose sparse activity encodes sensory information, each make multiple en passant synapses to MB output neurons (MBONs) in each compartment. Some MBONs have inputs from all KCs, while others differentially sample sensory modalities. Only 6% of KC>MBON synapses receive a direct synapse from a dopaminergic neuron (DAN). We identified two unanticipated classes of synapses, KC>DAN and DAN>MBON. DAN activation produces a slow depolarization of the MBON in these DAN>MBON synapses and can weaken memory recall.
Keywords: D. melanogaster; EM reconstruction; dopaminergic neuron; memory recall; mushroom body; neuroscience.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures








References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

