Melanin and Melanin-Related Polymers as Materials with Biomedical and Biotechnological Applications-Cuttlefish Ink and Mussel Foot Proteins as Inspired Biomolecules
- PMID: 28718807
- PMCID: PMC5536049
- DOI: 10.3390/ijms18071561
Melanin and Melanin-Related Polymers as Materials with Biomedical and Biotechnological Applications-Cuttlefish Ink and Mussel Foot Proteins as Inspired Biomolecules
Abstract
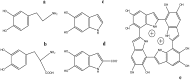
The huge development of bioengineering during the last years has boosted the search for new bioinspired materials, with tunable chemical, mechanical, and optoelectronic properties for the design of semiconductors, batteries, biosensors, imaging and therapy probes, adhesive hydrogels, tissue restoration, photoprotectors, etc. These new materials should complement or replace metallic or organic polymers that cause cytotoxicity and some adverse health effects. One of the most interesting biomaterials is melanin and synthetic melanin-related molecules. Melanin has a controversial molecular structure, dependent on the conditions of polymerization, and therefore tunable. It is found in animal hair and skin, although one of the common sources is cuttlefish (Sepia officinalis) ink. On the other hand, mussels synthesize adhesive proteins to anchor these marine animals to wet surfaces. Both melanin and mussel foot proteins contain a high number of catecholic residues, and their properties are related to these groups. Dopamine (DA) can easily polymerize to get polydopamine melanin (PDAM), that somehow shares properties with melanin and mussel proteins. Furthermore, PDAM can easily be conjugated with other components. This review accounts for the main aspects of melanin, as well as DA-based melanin-like materials, related to their biomedical and biotechnological applications.
Keywords: adhesive material; biofilms; biomedical applications; coated nanoparticles; melanin; polydopamine; therapeutical devices.
Conflict of interest statement
The author declares no conflict of interest.
Figures



References
-
- Solano F. Melanins: Skin pigments and much more-types, structural models, biological functions, and formation routes. New J. Sci. 2014;2014:498276. doi: 10.1155/2014/498276. - DOI
-
- Kutyrev A. Nucleophilic reactions of quinones. Tetrahedron. 1991;47:8043–8065. doi: 10.1016/S0040-4020(01)91002-6. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

