Tobacco Harm Reduction with Vaporised Nicotine (THRiVe): The Study Protocol of an Uncontrolled Feasibility Study of Novel Nicotine Replacement Products among People Living with HIV Who Smoke
- PMID: 28718828
- PMCID: PMC5551237
- DOI: 10.3390/ijerph14070799
Tobacco Harm Reduction with Vaporised Nicotine (THRiVe): The Study Protocol of an Uncontrolled Feasibility Study of Novel Nicotine Replacement Products among People Living with HIV Who Smoke
Abstract
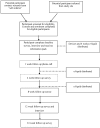
Smoking is a leading cause of morbidity and premature mortality among people living with HIV (PLHIV), who have high rates of tobacco smoking. Vaporised nicotine products (VNPs) are growing in popularity as a quit aid and harm reduction tool. However, little is known about their acceptability and use among PLHIV. Using a pragmatic, uncontrolled, mixed methods design this exploratory clinical trial aims to examine the feasibility of conducting a powered randomised clinical trial of VNPs as a smoking cessation and harm reduction intervention among vulnerable populations, such as PLHIV who smoke tobacco. Convenience sampling and snowball methods will be used to recruit participants (N = 30) who will receive two VNPs and up to 12 weeks' supply of nicotine e-liquid to use in a quit attempt. Surveys will be completed at weeks 0 (baseline), 4, 8, 12 (end of treatment) and 24 (end of the study) and qualitative interviews at weeks 0 and 12. As far as we are aware, this feasibility study is the first to trial VNPs among PLHIV for smoking cessation. If feasible and effective, this intervention could offer a new approach to reducing the high burden of tobacco-related disease among PLHIV and other vulnerable populations.
Keywords: HIV; VNPs; e-cigarettes; feasibility; harm reduction; smoking; tobacco; vaporised nicotine.
Conflict of interest statement
No other authors declare conflicts of interest. Mark Boyd has received research grant funding (paid to the institution) from AbbVie, Gilead and Merck and received honoraria for participation in HIV Advisory Boards and for the preparation and delivery of educational materials from AbbVie, Boehringer-Ingelheim, Bristol Myers Squibb, Gilead, Janssen-Cilag, Merck and ViiV Healthcare.
References
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials