Ante mortem cerebrospinal fluid tau levels correlate with postmortem tau pathology in frontotemporal lobar degeneration
- PMID: 28719018
- PMCID: PMC5776747
- DOI: 10.1002/ana.24996
Ante mortem cerebrospinal fluid tau levels correlate with postmortem tau pathology in frontotemporal lobar degeneration
Abstract
Objective: To test the hypotheses that (1) antemortem cerebrospinal fluid (CSF) tau levels correlate with postmortem tau pathology in frontotemporal lobar degeneration (FTLD) and (2) tauopathy patients have higher phosphorylated-tau levels compared to transactivation response element DNA-binding protein 43 (TDP-43) proteinopathy patients while accounting for Alzheimer's disease (AD) copathology.
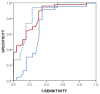
Methods: Patients had autopsy-confirmed FTLD with tauopathy (n = 31), TDP-43 proteinopathy (n = 49), or AD (n = 26) with antemortem CSF. CSF tau levels were compared between groups and correlated with digital histology measurement of postmortem tau pathology averaged from three cerebral regions (angular gyrus, mid-frontal cortex, and anterior cingulate gyrus). Multivariate linear regression tested the association of ante mortem CSF tau levels with postmortem tau pathology adjusting for demographics.
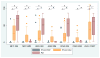
Results: Multivariate regression found an independent association of ante mortem CSF phosphorylated tau levels with postmortem cerebral tau pathology in FTLD (Beta = 1.3; 95% confidence interval = 0.2-2.4; p < 0.02). After excluding patients with coincident AD-associated tau pathology accompanying sporadic FTLD, we found lower CSF phosphorylated tau levels in the TDP-43 group (median = 7.4pg/ml; interquartile range [IQR] = 6.0, 12.3; n = 26) compared to the tauopathy group (median = 12.5pg/ml; IQR = 10.7, 15.0; n = 23; Z = 2.6; p < 0.01).
Interpretation: CSF phosphorylated-tau levels are positively associated with cerebral tau burden in FTLD. In vivo detection of AD copathology in sporadic FTLD patients may help stratify clinical cohorts with pure neuropathology in which low CSF phosphorylated-tau levels may have diagnostic utility to distinguish TDP-43 proteinopathy from tauopathy. Autopsy-confirmed samples are critical for FTLD biomarker development and validation. Ann Neurol 2017;82:247-258.
© 2017 American Neurological Association.
Conflict of interest statement
None.
Figures



References
-
- Tapiola T, Alafuzoff I, Herukka SK, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Archives of neurology. 2009 Mar;66(3):382–9. - PubMed
-
- Buerger K, Ewers M, Pirttila T, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain : a journal of neurology. 2006 Nov;129(Pt 11):3035–41. - PubMed
-
- Otto M, Wiltfang J, Tumani H, et al. Elevated levels of tau-protein in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Neuroscience letters. 1997 Apr 11;225(3):210–2. - PubMed
-
- Ost M, Nylen K, Csajbok L, et al. Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology. 2006 Nov 14;67(9):1600–4. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

