Phase 1 Randomized Study of a Tetravalent Dengue Purified Inactivated Vaccine in Healthy Adults in the United States
- PMID: 28719287
- PMCID: PMC5462566
- DOI: 10.4269/ajtmh.16-0634
Phase 1 Randomized Study of a Tetravalent Dengue Purified Inactivated Vaccine in Healthy Adults in the United States
Abstract
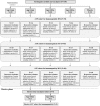
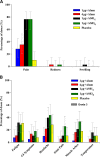
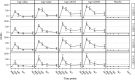
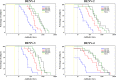
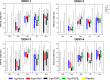
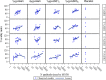
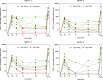
AbstractThe safety and immunogenicity of four formulations of an investigational tetravalent dengue purified inactivated vaccine (DPIV), formulated at 1 or 4 μg with aluminum hydroxide (alum) or at 1 μg with an adjuvant system (AS01E or AS03B), were evaluated in a first-time-in-human, placebo-controlled, randomized, observer-blind, phase 1 trial in the continental United States. Two doses of vaccine or placebo were administered intramuscularly 4 weeks apart to 100 healthy adults 18-39 years of age, randomized 1:1:1:1:1 to receive one of four DPIV formulations or saline placebo. The response to a third dose was evaluated in a subset of nine participants remote from primary vaccination. Humoral immunogenicity was assessed using a 50% microneutralization assay. All DPIV formulations were well tolerated. No vaccine-related serious adverse events were observed through 12 months after the second vaccine dose. In all DPIV groups, geometric mean antibody titers peaked at Day 56, waned through 6 months after the second vaccine dose, and then stabilized. In the nine subjects where boosting was evaluated, a strong anamnestic response was observed. These results support continuation of the clinical development of this dengue vaccine candidate (clinicaltrials.gov: NCT01666652).
Conflict of interest statement
Disclosure: Alix Collard, Edith Lepine, Jean-François Toussaint, Alexander C. Schmidt, and Bruce Innis are employed by the GSK group of companies. Edith Lepine, Alexander C. Schmidt, Bruce Innis, and Jean-François Toussaint also declare owing stocks, stock options, and/or restricted shares. Leyi Lin, Luis J. Martinez, Rafael De La Barrera, Richard G. Jarman, Kristopher Paolino, and Richard C. Ruck have nothing to disclose. Kenneth H. Eckels has an issued and licensed patent DENV PIV Vaccine with royalties paid to GSK. Stephen J. Thomas declares having received travel support as part of the cooperative agreement between the GSK group of companies and U.S. Army, which was put in place to codevelop the dengue vaccine candidate being reported. These statements are made in the interest of full disclosure and not because the authors consider this to be a conflict of interest.
Figures

Similar articles
-
Phase I Randomized Study of a Tetravalent Dengue Purified Inactivated Vaccine in Healthy Adults from Puerto Rico.Am J Trop Med Hyg. 2018 May;98(5):1435-1443. doi: 10.4269/ajtmh.17-0627. Epub 2018 Mar 1. Am J Trop Med Hyg. 2018. PMID: 29512481 Free PMC article. Clinical Trial.
-
Safety and Immunogenicity of an AS03B-Adjuvanted Inactivated Tetravalent Dengue Virus Vaccine Administered on Varying Schedules to Healthy U.S. Adults: A Phase 1/2 Randomized Study.Am J Trop Med Hyg. 2020 Jul;103(1):132-141. doi: 10.4269/ajtmh.19-0738. Epub 2020 Apr 23. Am J Trop Med Hyg. 2020. PMID: 32342848 Free PMC article. Clinical Trial.
-
Safety and Immunogenicity of Different Formulations of a Tetravalent Dengue Purified Inactivated Vaccine in Healthy Adults from Puerto Rico: Final Results after 3 Years of Follow-Up from a Randomized, Placebo-Controlled Phase I Study.Am J Trop Med Hyg. 2020 May;102(5):951-954. doi: 10.4269/ajtmh.19-0461. Am J Trop Med Hyg. 2020. PMID: 32124728 Free PMC article. Clinical Trial.
-
Development of a recombinant, chimeric tetravalent dengue vaccine candidate.Vaccine. 2015 Dec 10;33(50):7112-20. doi: 10.1016/j.vaccine.2015.11.022. Epub 2015 Nov 14. Vaccine. 2015. PMID: 26585500 Review.
-
Dengue vaccine candidates in development.Curr Top Microbiol Immunol. 2010;338:129-43. doi: 10.1007/978-3-642-02215-9_10. Curr Top Microbiol Immunol. 2010. PMID: 19802583 Review.
Cited by
-
Recent Developments in Recombinant Protein-Based Dengue Vaccines.Front Immunol. 2018 Aug 23;9:1919. doi: 10.3389/fimmu.2018.01919. eCollection 2018. Front Immunol. 2018. PMID: 30190720 Free PMC article. Review.
-
Enhanced Immunogenicity of Inactivated Dengue Vaccines by Novel Polysaccharide-Based Adjuvants in Mice.Microorganisms. 2022 May 16;10(5):1034. doi: 10.3390/microorganisms10051034. Microorganisms. 2022. PMID: 35630476 Free PMC article.
-
Challenges in Dengue Vaccines Development: Pre-existing Infections and Cross-Reactivity.Front Immunol. 2020 Jun 16;11:1055. doi: 10.3389/fimmu.2020.01055. eCollection 2020. Front Immunol. 2020. PMID: 32655548 Free PMC article. Review.
-
Phase I Randomized Study of a Tetravalent Dengue Purified Inactivated Vaccine in Healthy Adults from Puerto Rico.Am J Trop Med Hyg. 2018 May;98(5):1435-1443. doi: 10.4269/ajtmh.17-0627. Epub 2018 Mar 1. Am J Trop Med Hyg. 2018. PMID: 29512481 Free PMC article. Clinical Trial.
-
Vaccine development for mosquito-borne viral diseases.Front Immunol. 2023 May 12;14:1161149. doi: 10.3389/fimmu.2023.1161149. eCollection 2023. Front Immunol. 2023. PMID: 37251387 Free PMC article. Review.
References
-
- World Health Organization . Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. new edition. Geneva, Switzerland: World Health Organization; 2009. - PubMed
-
- Murrell S, Wu SC, Butler M. Review of dengue virus and the development of a vaccine. Biotechnol Adv. 2011;29:239–247. - PubMed
-
- Yauch LE, Shresta S. Dengue virus vaccine development. Adv Virus Res. 2014;88:315–372. - PubMed
-
- Screaton G, Mongkolsapaya J, Yacoub S, Roberts C. New insights into the immunopathology and control of dengue virus infection. Nat Rev Immunol. 2015;15:745–759. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

