Effect of High-Dose vs Standard-Dose Wintertime Vitamin D Supplementation on Viral Upper Respiratory Tract Infections in Young Healthy Children
- PMID: 28719693
- PMCID: PMC5817430
- DOI: 10.1001/jama.2017.8708
Effect of High-Dose vs Standard-Dose Wintertime Vitamin D Supplementation on Viral Upper Respiratory Tract Infections in Young Healthy Children
Abstract
Importance: Epidemiological studies support a link between low 25-hydroxyvitamin D levels and a higher risk of viral upper respiratory tract infections. However, whether winter supplementation of vitamin D reduces the risk among children is unknown.
Objective: To determine whether high-dose vs standard-dose vitamin D supplementation reduces the incidence of wintertime upper respiratory tract infections in young children.
Design, setting, and participants: A randomized clinical trial was conducted during the winter months between September 13, 2011, and June 30, 2015, among children aged 1 through 5 years enrolled in TARGet Kids!, a multisite primary care practice-based research network in Toronto, Ontario, Canada.
Interventions: Three hundred forty-nine participants were randomized to receive 2000 IU/d of vitamin D oral supplementation (high-dose group) vs 354 participants who were randomized to receive 400 IU/d (standard-dose group) for a minimum of 4 months between September and May.
Main outcome measures: The primary outcome was the number of laboratory-confirmed viral upper respiratory tract infections based on parent-collected nasal swabs over the winter months. Secondary outcomes included the number of influenza infections, noninfluenza infections, parent-reported upper respiratory tract illnesses, time to first upper respiratory tract infection, and serum 25-hydroxyvitamin D levels at study termination.
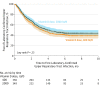
Results: Among 703 participants who were randomized (mean age, 2.7 years, 57.7% boys), 699 (99.4%) completed the trial. The mean number of laboratory-confirmed upper respiratory tract infections per child was 1.05 (95% CI, 0.91-1.19) for the high-dose group and 1.03 (95% CI, 0.90-1.16) for the standard-dose group, for a between-group difference of 0.02 (95% CI, -0.17 to 0.21) per child. There was no statistically significant difference in number of laboratory-confirmed infections between groups (incidence rate ratio [RR], 0.97; 95% CI, 0.80-1.16). There was also no significant difference in the median time to the first laboratory-confirmed infection: 3.95 months (95% CI, 3.02-5.95 months) for the high-dose group vs 3.29 months (95% CI, 2.66-4.14 months) for the standard-dose group, or number of parent-reported upper respiratory tract illnesses between groups (625 for high-dose vs 600 for standard-dose groups, incidence RR, 1.01; 95% CI, 0.88-1.16). At study termination, serum 25-hydroxyvitamin D levels were 48.7 ng/mL (95% CI, 46.9-50.5 ng/mL) in the high-dose group and 36.8 ng/mL (95% CI, 35.4-38.2 ng/mL) in the standard-dose group.
Conclusions and relevance: Among healthy children aged 1 to 5 years, daily administration of 2000 IU compared with 400 IU of vitamin D supplementation did not reduce overall wintertime upper respiratory tract infections. These findings do not support the routine use of high-dose vitamin D supplementation in children for the prevention of viral upper respiratory tract infections.
Trial registration: clinicaltrials.gov Identifier: NCT01419262.
Conflict of interest statement
Figures

Comment in
-
Vitamin D Supplementation and Upper Respiratory Tract Infections in Children.JAMA. 2017 Dec 5;318(21):2138-2139. doi: 10.1001/jama.2017.15150. JAMA. 2017. PMID: 29209712 No abstract available.
References
-
- Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163(4):487-494. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

