Association of the FDA Amendment Act with trial registration, publication, and outcome reporting
- PMID: 28720112
- PMCID: PMC5516301
- DOI: 10.1186/s13063-017-2068-3
Association of the FDA Amendment Act with trial registration, publication, and outcome reporting
Abstract
Background: Selective clinical trial publication and outcome reporting has the potential to bias the medical literature. The 2007 Food and Drug Administration (FDA) Amendment Act (FDAAA) mandated clinical trial registration and outcome reporting on ClinicalTrials.gov, a publicly accessible trial registry.
Methods: Using publicly available data from ClinicalTrials.gov, FDA documents, and PubMed, we determined registration, publication, and reporting of findings for all efficacy trials supporting FDA approval of new drugs for cardiovascular disease and diabetes between 2005 and 2014, before and after the FDAAA. For published trials, we compared the published interpretation of the findings (positive, equivocal, or negative) with the FDA reviewer's interpretation.
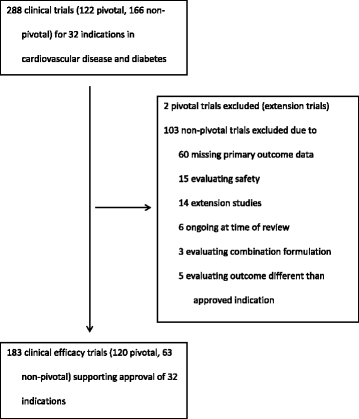
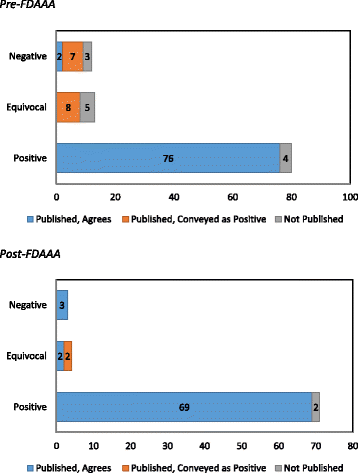
Results: Between 2005 and 2014, the FDA approved 30 drugs for 32 indications of cardiovascular disease (n = 17) and diabetes (n = 15) on the basis of 183 trials (median per indication 5.7 (IQR, 3-8)). Compared with pre FDAAA, post-FDAAA studies were more likely to be registered (78 of 78 (100%) vs 73 of 105 (70%); p < 0.001), to be published (76 of 78 (97%) vs 93 of 105 (89%); p = 0.03), and to present findings concordant with the FDA reviewer's interpretation (74 of 76 (97%) vs 78 of 93 (84%); p = 0.004). Pre FDAAA, the FDA reviewer interpreted 80 (76%) trials as positive and 91 (98%) were published as positive. Post FDAAA, the FDA reviewer interpreted 71 (91%) trials as positive and 71 (93%) were published as positive.
Conclusions: FDAAA was associated with increased registration, publication, and FDA-concordant outcome reporting for trials supporting FDA approval of new drugs for cardiovascular disease and diabetes.
Keywords: Clinical trials; Drug approval; Publications; United States Food and Drug Administration.
Conflict of interest statement
Ethics approval and consent to participate
Because our examination of trial publications did not involve human subjects, ethics committee review was not required by the Yale University Human Research Protection Program. Consent to participate not applicable.
Consent for publication
Not applicable.
Competing interests
NRD, HMK, and JSR receive support through Yale University from Johnson and Johnson to develop methods of clinical trial data sharing, from the Blue Cross Blue Shield Association to better understand medical technology evaluation, and from the Centers of Medicare and Medicaid Services (CMS) to develop and maintain performance measures that are used for public reporting. HMK and JSR receive support through Yale University from Medtronic, Inc. and the US FDA to develop methods for postmarket surveillance of medical devices. HMK, JEM, and JSR receive support through Yale University and New York University, respectively, from the Laura and John Arnold Foundation to better understand clinical research integrity and transparency. HMK reports that he chairs a scientific advisory board for UnitedHealthcare. ATP and CXZ declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- New Drugs: Grounds for Refusing Application; Approval of Application; “Substantial Evidence” Defined, 21 USC §355d. https://www.gpo.gov/fdsys/pkg/USCODE-2010-title21/html/USCODE-2010-title.... Accessed Aug 2016.
-
- Guidance for Industry: Providing Clinical Evidence of Effectiveness for Human Drug and Biological Products. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati.... Accessed Aug 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

