Profiling of the perturbed metabolomic state of mouse spleen during acute and chronic toxoplasmosis
- PMID: 28720125
- PMCID: PMC5516376
- DOI: 10.1186/s13071-017-2282-6
Profiling of the perturbed metabolomic state of mouse spleen during acute and chronic toxoplasmosis
Abstract
Background: Toxoplasma gondii, a common opportunistic protozoan, is a leading cause of illness and mortality among immunosuppressed individuals and during congenital infections. Current therapeutic strategies for toxoplasmosis are not fully effective at curtailing disease progression in these cases. Given the parasite ability to influence host immunity and metabolism, understanding of the metabolic alterations in the host's immune organs during T. gondii infection may enhance the understanding of the molecular mechanisms that define the pathophysiology of T. gondii infection.
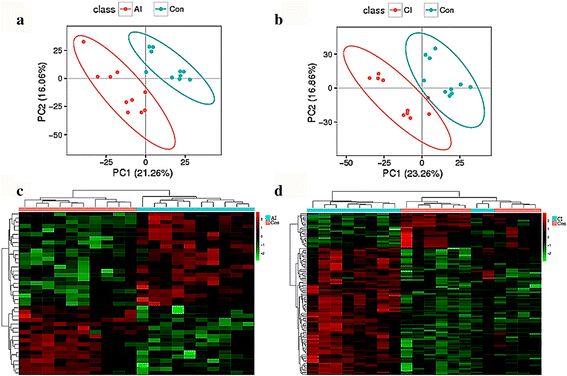
Methods: We investigated the global metabolic changes in the spleen of BALB/c mice at early and late stage of infection with T. gondii using LC-MS/MS-based metabolomics. Multivariate data analysis methods, principal components analysis (PCA) and partial least squares discriminant analysis (PLS-DA), were used to identify metabolites that are influenced by T. gondii infection.
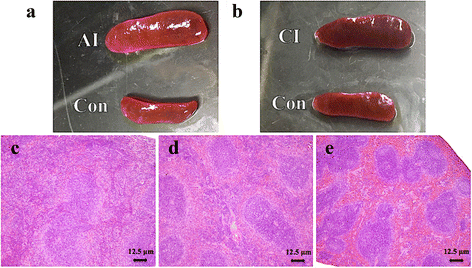
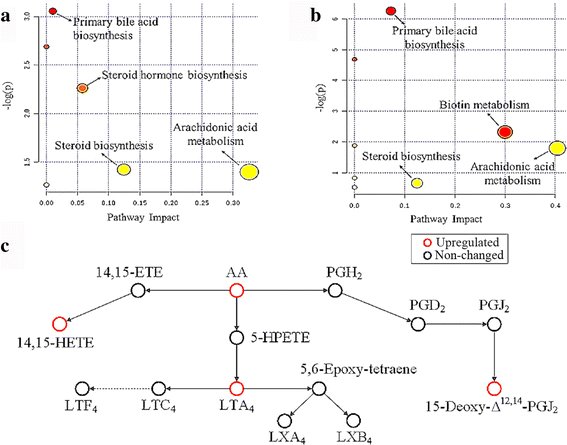
Results: Multivariate analyses clearly separated the metabolites of spleen of infected and control mice. A total of 132 differential metabolites were identified, 23 metabolites from acutely infected versus control mice and 109 metabolites from chronically infected versus control mice. Lipids, hormones, lactones, acids, peptides, antibiotics, alkaloids and natural toxins were the most influenced chemical groups. There were 12 shared differential metabolites between acutely infected versus control mice and chronically infected versus control mice, of which 4,4-Dimethyl-5alpha-cholesta-8,14,24-trien-3beta-ol was significantly upregulated and ubiquinone-8 was significantly downregulated. Major perturbed metabolic pathways included primary bile acid biosynthesis, steroid hormone biosynthesis, biotin metabolism, and steroid biosynthesis, with arachidonic acid metabolism being the most significantly impacted pathway. These metabolic changes suggest a multifactorial nature of the immunometabolic responses of mouse spleen to T. gondii infection.
Conclusions: This study demonstrated that T. gondii infection can cause significant metabolomic alterations in the spleen of infected mice. These findings provide new insights into the molecular mechanisms that underpin the pathogenesis of T. gondii infection.
Keywords: Mass spectrometry; Metabolome; Non-targeted metabolomics; Pathway enrichment analysis; Spleen; Toxoplasma gondii.
Conflict of interest statement
Ethics approval
All experiments were conducted with the approval of the Animal Administration and Ethics Committee of Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences (Permit No. LVRIAEC2016–007). All animals were handled in strict accordance with good laboratory animal practice according to the Animal Ethics Procedures and Guidelines of the People’s Republic of China. All efforts were made to minimize animal suffering.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Elsheikha HM. Congenital toxoplasmosis: priorities for further health promotion action. Public Health. 2008;122:335–53. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

