Efficacy and safety of direct switch to indacaterol/glycopyrronium in patients with moderate COPD: the CRYSTAL open-label randomised trial
- PMID: 28720132
- PMCID: PMC5516383
- DOI: 10.1186/s12931-017-0622-x
Efficacy and safety of direct switch to indacaterol/glycopyrronium in patients with moderate COPD: the CRYSTAL open-label randomised trial
Abstract
Background: Dual bronchodilation combining a long-acting β2-agonist (LABA) and a long-acting muscarinic antagonist (LAMA) is the preferred choice of treatment recommended by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 guidelines for the management of patients with moderate-to-severe chronic obstructive pulmonary disease (COPD). The once-daily (q.d.) fixed-dose combination (FDC) of LABA, indacaterol 110 μg and LAMA, glycopyrronium 50 μg (IND/GLY 110/50 μg q.d.) demonstrated superior improvements in lung function, dyspnoea and overall health status and better tolerability against LABA or LAMA monotherapies and combination of LABA and inhaled corticosteroid (ICS) in more than 11,000 patients with moderate-to-severe COPD in several randomised controlled clinical trials.
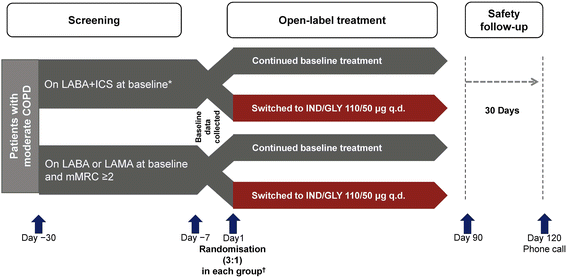
Methods: The CRYSTAL study was the first, 12-week, randomised, open-label trial that evaluated the efficacy and safety of a direct switch from previous treatments to IND/GLY 110/50 μg q.d. on lung function and dyspnoea in patients with moderate COPD and a history of up to one exacerbation in the previous year. Patients were divided into 2 groups according to their background therapy and symptom scores and were randomised (3:1) to IND/GLY or to continue with their previous treatments.
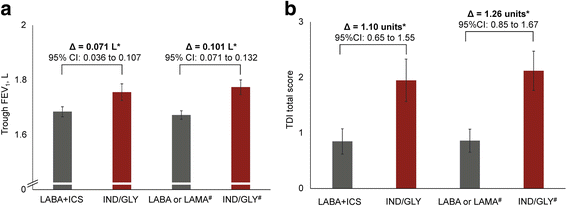
Results: The study included 4389 randomised patients, of whom 2160 were in groups switched to IND/GLY (intention-to-treat population). The effect of IND/GLY was superior to LABA + ICS on trough forced expiratory volume in 1 s (FEV1; treatment difference, Δ = +71 mL) and transition dyspnoea index (TDI; [Δ = 1.09 units]), and to LABA or LAMA on trough FEV1 (Δ = +101 mL) and a TDI (Δ = 1.26 units). Improvements in health status and lower rescue medication use were also observed with IND/GLY. The safety profile of the study medication was similar to that observed in previous studies.
Conclusions: IND/GLY demonstrated superior improvements in lung function and dyspnoea after direct switch from previous treatments.
Trial registration: ClinicalTrials.gov number: NCT01985334 .
Keywords: Chronic obstructive pulmonary disease; Direct switch; Dual bronchodilation; Indacaterol/glycopyrronium; Open-label.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol and all amendments were reviewed by an Independent Ethics Committee (IEC) or an Institutional Review Board (IRB) for each centre, in each country (Austria, Belgium, Czech Republic, Denmark, Estonia, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Norway, Poland, Portugal, Romania, Russian Federation, Slovakia [Slovak Republic], Slovenia, Spain, Sweden and UK). The study was conducted according to the ethical principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
Claus F. Vogelmeier (CFV) has received personal fees from Novartis during the conduct of the study. Outside the submitted work, he has received personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Grifols, Menarini, Mundipharma and Teva and grants from GlaxoSmithKline and Grifols. Mina Gaga (MG) has received grant and personal fees from Novartis, Pharmaten and Menarini, outside the submitted work. She has also received personal fees from Chiesi, Boehringer Ingelheim and Teva, received compensation for organising or participating in advisory boards from GlaxoSmithKline and received a grant from AstraZeneca. Maryam Aalamian-Mattheis (MA-M) is an employee of Novartis. Timm Greulich (TG) has received compensation from Novartis during the conduct of the study. He has also received lecture fees from AstraZeneca, Chiesi, CSL-Behring, GlaxoSmithKline, Grifols, Mundipharma and Novartis, received compensation for organising or participating in advisory boards from AstraZeneca, CSL-Behring, Novartis, Boehringer Ingelheim and Grifols and received a grant to support an AATD-Lab from Grifols. Jose M. Marin (JMM) does not have anything to disclose. Walter Castellani (WC) has received compensation from Novartis during the conduct of the study. Vincent Ninane (VN) has received compensation for organising or participating in advisory boards and lecture fees from Novartis, AstraZeneca, Boehringer Ingelheim and MSD, outside the submitted work. Stephen Lane (SL) has received speaker fees from Novartis, Menarini and Mundipharma, received unrestricted educational grant from Novartis during the conduct of the study and received compensation for organising or participating in advisory boards from GlaxoSmithKline, ALK Albello and Teva. Xavier Nunez (XN) was the statistician for the CRYSTAL study and works at a Novartis-contracted CRO. Francesco Patalano (FP), Andreas Clemens (AC) and Konstantinos Kostikas (KK) are employees and shareholders of Novartis Pharma AG. KK has received honoraria for speeches and/or consulting services from AstraZeneca, Boehringer Ingelheim, Chiesi, ELPEN and Takeda, outside the submitted work. None of the authors received any compensation for the development of the manuscript.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. Available from www.goldcopd.org. Accessed on 4 May 2017
-
- Vogelmeier CF, Bateman ED, Pallante J, Alagappan VK, D'Andrea P, Chen H, Banerji D. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1:51–60. doi: 10.1016/S2213-2600(12)70052-8. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

