Socioeconomic predictors of human papillomavirus vaccination in Danish men - A nationwide study
- PMID: 28720452
- PMCID: PMC5883232
- DOI: 10.1016/j.pvr.2016.11.004
Socioeconomic predictors of human papillomavirus vaccination in Danish men - A nationwide study
Abstract
Background: The quadrivalent human papillomavirus vaccine was licensed in Denmark in 2006. Unlike women, men are not offered human papillomavirus vaccination free of charge but can have it at their own expense. We investigated human papillomavirus vaccine uptake by men in Denmark and the socioeconomic factors that may predict human papillomavirus vaccination.
Methods: Using the Civil Registration System, we identified all boys and men aged 9-26 years in 2006-2013 and their mothers. By linkage to Statistics Denmark and the National Prescription Registry, we obtained information on socioeconomic variables and human papillomavirus vaccination during the study period. Using Cox regression, we examined the associations between socioeconomic variables and human papillomavirus vaccination.
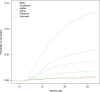
Results: Between 2006 and 2013, 6253 (0.8%) males aged 9-26 years were vaccinated against human papillomavirus. The strongest predictor identified was ethnicity. Males who were immigrants (hazard ratio, 0.12; 95% confidence interval, 0.08-0.180) or sons of immigrant parents (hazard ratio, 0.13; 95% confidence interval, 0.10-0.17) were less likely to be vaccinated than Danish males. Additionally, sons of mothers who were unemployed, unmarried, had a low income, and basic education initiated human papillomavirus vaccination less frequently. Finally, sons of mothers who were physicians or nurses were more likely to be vaccinated than sons of other highly educated mothers.
Conclusion: We found low uptake, with social disparities in human papillomavirus vaccination of boys and young men in Denmark.
Keywords: Human papillomavirus; Men; Nationwide; Uptake; Vaccination.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Globocan 2012. Available at: 〈http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx〉 (accessed 20.08.15), Ref Type: Online Source
-
- Herrero R., Gonzalez P., Markowitz L.E. Present status of human papillomavirus vaccine development and implementation. Lancet Oncol. 2015;16(5):e206–e216. - PubMed
-
- European Medicines Agency. Gardasil. Available at: 〈http://www.ema.europa.eu/ema/index.jsp?Curl=pages/medicines/human/medic... (accessed 8.07.15), Ref Type: Online Source
-
- Food and Drug Administration Gardasil. Available at: 〈http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm... (accessed 8.05.15) Ref Type: Online Source
LinkOut - more resources
Full Text Sources
Other Literature Sources

