Catalysis and chemical mechanisms of calcite dissolution in seawater
- PMID: 28720698
- PMCID: PMC5547618
- DOI: 10.1073/pnas.1703604114
Catalysis and chemical mechanisms of calcite dissolution in seawater
Abstract
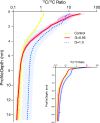
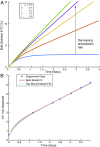
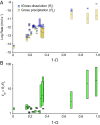
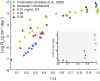
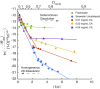
Near-equilibrium calcite dissolution in seawater contributes significantly to the regulation of atmospheric [Formula: see text] on 1,000-y timescales. Despite many studies on far-from-equilibrium dissolution, little is known about the detailed mechanisms responsible for calcite dissolution in seawater. In this paper, we dissolve 13C-labeled calcites in natural seawater. We show that the time-evolving enrichment of [Formula: see text] in solution is a direct measure of both dissolution and precipitation reactions across a large range of saturation states. Secondary Ion Mass Spectrometer profiles into the 13C-labeled solids confirm the presence of precipitated material even in undersaturated conditions. The close balance of precipitation and dissolution near equilibrium can alter the chemical composition of calcite deeper than one monolayer into the crystal. This balance of dissolution-precipitation shifts significantly toward a dissolution-dominated mechanism below about [Formula: see text] Finally, we show that the enzyme carbonic anhydrase (CA) increases the dissolution rate across all saturation states, and the effect is most pronounced close to equilibrium. This finding suggests that the rate of hydration of [Formula: see text] is a rate-limiting step for calcite dissolution in seawater. We then interpret our dissolution data in a framework that incorporates both solution chemistry and geometric constraints on the calcite solid. Near equilibrium, this framework demonstrates a lowered free energy barrier at the solid-solution interface in the presence of CA. This framework also indicates a significant change in dissolution mechanism at [Formula: see text], which we interpret as the onset of homogeneous etch pit nucleation.
Keywords: catalysis; isotope geochemistry; mineral dissolution; oceanography.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Feely RA. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science. 2004;305:362–366. - PubMed
-
- Archer D, Kheshgi H, Maier-Reimer E. Dynamics of fossil fuel CO2 neutralization by marine CaCO3. Global Biogeochem Cycles. 1998;12:259–276.
-
- Ilyina T, Zeebe RE. Detection and projection of carbonate dissolution in the water column and deep-sea sediments due to ocean acidification. Geophys Res Lett. 2012;39:L06606.
-
- Honjo S, Erez J. Dissolution rates of calcium carbonate in the deep ocean; an in-situ experiment in the North Atlantic Ocean. Earth Planet Sci Lett. 1978;40:287–300.
-
- Walter LM, Morse JW. The dissolution kinetics of shallow marine carbonates in seawater: A laboratory study. Geochim Cosmochim Acta. 1985;49:1503–1513.
LinkOut - more resources
Full Text Sources
Other Literature Sources

