Conceptual framework of the eco-physiological phases of insect diapause development justified by transcriptomic profiling
- PMID: 28720705
- PMCID: PMC5559046
- DOI: 10.1073/pnas.1707281114
Conceptual framework of the eco-physiological phases of insect diapause development justified by transcriptomic profiling
Abstract
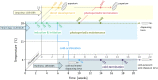
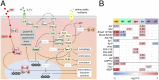
Insects often overcome unfavorable seasons in a hormonally regulated state of diapause during which their activity ceases, development is arrested, metabolic rate is suppressed, and tolerance of environmental stress is bolstered. Diapausing insects pass through a stereotypic succession of eco-physiological phases termed "diapause development." The phasing is varied in the literature, and the whole concept is sometimes criticized as being too artificial. Here we present the results of transcriptional profiling using custom microarrays representing 1,042 genes in the drosophilid fly, Chymomyza costata Fully grown, third-instar larvae programmed for diapause by a photoperiodic (short-day) signal were assayed as they traversed the diapause developmental program. When analyzing the gradual dynamics in the transcriptomic profile, we could readily distinguish distinct diapause developmental phases associated with induction/initiation, maintenance, cold acclimation, and termination by cold or by photoperiodic signal. Accordingly, each phase is characterized by a specific pattern of gene expression, supporting the physiological relevance of the concept of diapause phasing. Further, we have dissected in greater detail the changes in transcript levels of elements of several signaling pathways considered critical for diapause regulation. The phase of diapause termination is associated with enhanced transcript levels in several positive elements stimulating direct development (the 20-hydroxyecdysone pathway: Ecr, Shd, Broad; the Wnt pathway: basket, c-jun) that are countered by up-regulation in some negative elements (the insulin-signaling pathway: Ilp8, PI3k, Akt; the target of rapamycin pathway: Tsc2 and 4EBP; the Wnt pathway: shaggy). We speculate such up-regulations may represent the early steps linked to termination of diapause programming.
Keywords: development; diapause; insects; microarrays; transcriptomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Lees AD. The physiology and biochemistry of diapause. Annu Rev Entomol. 1956;1:1–16.
-
- Hodek I. Role of environmental factors and endogenous mechanisms in the seasonality of reproduction in insects diapausing as adults. In: Brown VK, Hodek I, editors. Diapause and Life Cycle Strategies in Insects. Dr W Junk Publishers; The Hague: 1983. pp. 9–33.
-
- Tauber MJ, Tauber CA, Masaki S. Seasonal Adaptations of Insects. Oxford Univ Press; Oxford, UK: 1986. p. 411.
-
- Danks HV. 1987. Insect dormancy: An ecological perspective. Terrestrial Arthropods, Monograph series No. 1 (National Museum of Natural Sciences, Ottawa) p 439.
-
- Saunders DS. In: Insect Clocks. Steel CGH, Vafopoulou X, Lewis RD, editors. Elsevier; Amsterdam: 2002. p. 576.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

