Commensal Gut Microbiota Immunomodulatory Actions in Bone Marrow and Liver have Catabolic Effects on Skeletal Homeostasis in Health
- PMID: 28720797
- PMCID: PMC5515851
- DOI: 10.1038/s41598-017-06126-x
Commensal Gut Microbiota Immunomodulatory Actions in Bone Marrow and Liver have Catabolic Effects on Skeletal Homeostasis in Health
Abstract
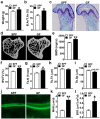
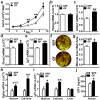
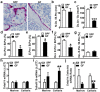
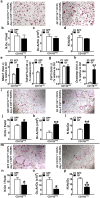
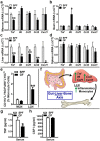
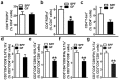
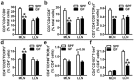
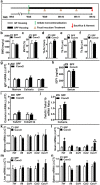
Despite knowledge the gut microbiota regulates bone mass, mechanisms governing the normal gut microbiota's osteoimmunomodulatory effects on skeletal remodeling and homeostasis are unclear in the healthy adult skeleton. Young adult specific-pathogen-free and germ-free mice were used to delineate the commensal microbiota's immunoregulatory effects on osteoblastogenesis, osteoclastogenesis, marrow T-cell hematopoiesis, and extra-skeletal endocrine organ function. We report the commensal microbiota has anti-anabolic effects suppressing osteoblastogenesis and pro-catabolic effects enhancing osteoclastogenesis, which drive bone loss in health. Suppression of Sp7(Osterix) and Igf1 in bone, and serum IGF1, in specific-pathogen-free mice suggest the commensal microbiota's anti-osteoblastic actions are mediated via local disruption of IGF1-signaling. Differences in the RANKL/OPG Axis in vivo, and RANKL-induced maturation of osteoclast-precursors in vitro, indicate the commensal microbiota induces sustained changes in RANKL-mediated osteoclastogenesis. Candidate mechanisms mediating commensal microbiota's pro-osteoclastic actions include altered marrow effector CD4+T-cells and a novel Gut-Liver-Bone Axis. The previously unidentified Gut-Liver-Bone Axis intriguingly implies the normal gut microbiota's osteoimmunomodulatory actions are partly mediated via immunostimulatory effects in the liver. The molecular underpinnings defining commensal gut microbiota immunomodulatory actions on physiologic bone remodeling are highly relevant in advancing the understanding of normal osteoimmunological processes, having implications for the prevention of skeletal deterioration in health and disease.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

