Bivalent DNA vaccine induces significant immune responses against infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus in rainbow trout
- PMID: 28720888
- PMCID: PMC5515949
- DOI: 10.1038/s41598-017-06143-w
Bivalent DNA vaccine induces significant immune responses against infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus in rainbow trout
Abstract
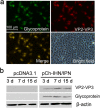
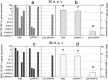
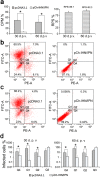
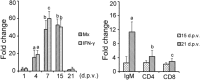
Infectious hematopoietic necrosis virus (IHNV) and infectious pancreatic necrosis virus (IPNV) are important pathogens of salmon and trout. An active bivalent DNA vaccine was constructed with the glycoprotein gene of Chinese IHNV isolate Sn1203 and VP2-VP3 gene of Chinese IPNV isolate ChRtm213. Rainbow trout (5 g) were vaccinated by intramuscular injection with 1.0 µg of the bivalent DNA vaccine and then challenged with an intraperitoneal injection of IHNV, IPNV, or both, at 30 and 60 days post-vaccination (d.p.v.). High protection rates against IHNV were observed, with 6% and 10% cumulative mortality, respectively, compared with 90-94% in the mock-vaccinated groups. IPNV loads (531-fold and 135-fold, respectively) were significantly reduced in the anterior kidneys of the vaccinated trout. Significant protection against co-infection with IHNV and IPNV was observed, with cumulative mortality rates of 6.67% and 3.33%, respectively, compared with 50.0% and 43.3%, respectively, in the mock-vaccinated groups. No detectable infective IHNV or IPNV was recovered from vaccinated trout co-infected with IHNV and IPNV. The bivalent DNA vaccine increased the expression of Mx-1 and IFN-γ at 4, 7, and 15 d.p.v, and IgM at 21 d.p.v., and induced high titres (≥160) of IHNV and IPNV neutralizing antibodies at 30 and 60 d.p.v.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

