Synthesis, construction, and evaluation of self-assembled nano-bacitracin A as an efficient antibacterial agent in vitro and in vivo
- PMID: 28721045
- PMCID: PMC5501637
- DOI: 10.2147/IJN.S136998
Synthesis, construction, and evaluation of self-assembled nano-bacitracin A as an efficient antibacterial agent in vitro and in vivo
Erratum in
-
Erratum: Synthesis, construction, and evaluation of self-assembled nano-bacitracin A as an efficient antibacterial agent in vitro and in vivo [Corrigendum].Int J Nanomedicine. 2019 Jan 23;14:771. doi: 10.2147/IJN.S201516. eCollection 2019. Int J Nanomedicine. 2019. PMID: 30774331 Free PMC article.
-
Erratum: Synthesis, Construction, and Evaluation of Self-Assembled Nano-Bacitracin a as an Efficient Antibacterial Agent in vitro and in vivo [Corrigendum].Int J Nanomedicine. 2020 Jul 2;15:4753. doi: 10.2147/IJN.S267460. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32753863 Free PMC article.
Abstract
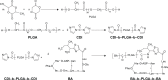
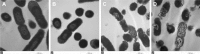
Bacitracin A (BA) is an excellent polypeptide antibiotic that is active against gram-positive bacteria without triggering multidrug resistance. However, BA is inactive against gram-negative bacteria because of its inability to cross the outer membrane of these cells, and it has strong nephrotoxicity, thus limiting its clinical applications. Nanoantibiotics can effectively localize antibiotics to the periplasmic space of bacteria while decreasing the adverse effects of antibiotics. In this study, biodegradable hydrophobic copolymers of poly (d,l-lactide-co-glycolide) (PLGA) were attached to the N-termini of BA to design a novel class of self-assembled nano-bacitracin A (nano-BAs), and their potential as antibacterial agents was evaluated in vitro and in vivo. Nano-BAs had a core-shell structure with a mean diameter <150 nm. Impressively, nano-BAs had strong antibacterial properties against both gram-positive and gram-negative bacteria, and the distribution of antibacterial activity as a function of PLGA block length was skewed toward longer PLGA chains. No cytotoxicity against HK-2 cells or human red blood cells (hRBCs) was observed in vitro, suggesting good biocompatibility. A high local density of BA mass on the surface promoted endocytotic cellular uptake, and hydrophobic interactions between the PLGA block and lipopolysaccharide (LPS) facilitated the uptake of nano-BAs, thereby leading to greater antibacterial activities. In addition, Nano-BA5K was found to be effective in vivo, and it served as an anti-infective agent for wound healing. Collectively, this study provides a cost-effective means of developing self-assembling nano-polypeptide antibiotic candidates with a broader antibacterial spectrum and a lower toxicity than commercially available peptide antibiotics, owing to their modification with biodegradable copolymers.
Keywords: bacitracin A; broader antibacterial spectrum; nano-BAs; poly (d,l-lactide-co-glycolide); wound healing.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures









References
-
- Meleney FL, Johnson BA. Bacitracin. Am J Med. 1949;7(6):794–806. - PubMed
-
- Johnson BA, Anker H, Meleney FL. Bacitracin: a new antibiotic produced by a member of the B. Subtilis Group. Science. 1945;102(2650):376–377. - PubMed
-
- Chapnick EK, Gradon JD, Kreiswirth B, et al. Comparative killing kinetics of methicillin-resistant Staphylococcus aureus by bacitracin or mupirocin. Infect Control Hosp Epidemiol. 1996;17(3):178–180. - PubMed
-
- Tsuji K, Robertson JH. Improved high-performance liquid chromatographic method for polypeptide antibiotics and its application to study the effects of treatments to reduce microbial levels in bacitracin powder. J Chromatogr. 1975;112:663–672. - PubMed
-
- Ming LJ, Epperson JD. Metal binding and structure-activity relationship of the metalloantibiotic peptide bacitracin. J Inorg Biochem. 2002;91(1):46–58. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

