Structural differences and architectural features of two different polypropylene slings (TVT-O and I-STOP) have no impact on biocompatibility and tissue reactions
- PMID: 28721282
- PMCID: PMC5510338
- DOI: 10.5173/ceju.2017.1189
Structural differences and architectural features of two different polypropylene slings (TVT-O and I-STOP) have no impact on biocompatibility and tissue reactions
Abstract
Introduction: To evaluate the impact of design features of the synthetic mid-urethral slings on tissue integrity and inflammatory responses.
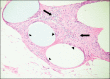
Material and methods: In total 30 female Sprague-Dawley rats were implanted with type I monofilamentous, macroporous polypropylene meshes: Gynecare TVT-Obturator tape® (Ethicon Inc., Johnson & Johnson, Somerville, NJ, USA) and I-STOP® (CL Medical Inc., Lyon, France). All animal groups were sacrificed at set time intervals - 6 weeks, 3 months, 6 months, 9 months and 12 months - and the abdominal wall was harvested with mesh strips for histological evaluation.
Results: All mesh strips appeared to be well incorporated into the abdominal wall, and no signs of shrinkage was noticed. All specimens showed a thin/delicate, loose, fibrous interface between the synthetic graft plate and abdominal wall, along with mild inflammatory reactions from 6 weeks to 12 months.
Conclusions: Both mesh brands induced comparable, minimal foreign body reactions and integrated well into the host tissues despite differences in architectural features. TVT-O® and I-STOP® evoked similar low-grade inflammatory responses up to 12 months in this animal model. Structural differences and architectural features of polypropylene slings used in this study have had no impact on tissue integrity and inflammatory responses.
Keywords: I-STOP; TVT; histological assessment; inflammatory response; mid-urethral sling; tissue reaction.
Conflict of interest statement
Drs. Przydacz, Adli, Mahfouz, Loutochin and Bégin have nothing to disclose. Dr. Corcos is a consultant for Astellas, Pfizer, Allergan, outside the submitted work.
Figures



References
-
- Abrams P, Cardozo L, Fall M, et al. Standardization Sub-committee of the International Continence Society. The standardization of terminology in lower urinary tract function: report from the Standardization Sub-committee of the International Continence Society. Urology. 2003;61:37–49. - PubMed
-
- Walker GJ, Gunasekera P. Pelvic organ prolapse and incontinence in developing countries: review of prevalence and risk factors. Int Urogynecol J. 2011;22:127–135. - PubMed
-
- Milsom I, Altman D, Lapitan MC, et al. Epidemiology of urinary (UI) and faecal (FI) incontinence and pelvic organ prolapse (POP) In: Abrams P, Cardozo L, Khoury S, Wein AJ, editors. Incontinence: 4th International Consultation on Incontinence. Paris, France: Health Publications Ltd.; 2009. pp. 35–112.
-
- Latthe PM, Singh P, Foon R, et al. Two routes of transobturator tape procedures in stress urinary incontinence: a meta-analysis with direct and indirect comparison of randomized trials. BJU Int. 2010;106:68–76. - PubMed
-
- Burkhard FC, Lucas MG, Berghmans LC, et al. European Association of Urology (EAU), EAU Guidelines on the Urinary Incontinence in Adults [Internet] Published: March 2016 [Accessed: September 2016] Available from: https://uroweb.org/guideline/urinary-incontinence.
LinkOut - more resources
Full Text Sources
Other Literature Sources
