Activin-A signaling promotes epithelial-mesenchymal transition, invasion, and metastatic growth of breast cancer
- PMID: 28721365
- PMCID: PMC5515205
- DOI: 10.1038/npjbcancer.2015.7
Activin-A signaling promotes epithelial-mesenchymal transition, invasion, and metastatic growth of breast cancer
Abstract
Background: Activins belong to the transforming growth factor-β (TGF-β) superfamily of cytokines. Although the role of TGF-β in cancer progression has been highly advocated, the role of activin signaling in cancer is not well known. However, overexpression of activin-A has been observed in several cancers.
Aims: The gene expression profile indicated higher expression of Activin-A in breast tumors. Hence the aim of this study was to evaluate the status and role of Activin signaling pathway in these tumors.
Methods: Microarray analysis was performed to reveal gene expression changes in breast tumors. The results were validated by quantitative PCR and immunohistochemical analysis in two independent sets of normal and tumor samples. Further, correlation of activin expression with survival and distant metastasis was performed to evaluate its possible role in tumor progression. We used recombinant activin-A, inhibitors, overexpression, and knockdown strategies both in vitro and in vivo, to understand the mechanism underlying the protumorigenic role of this signaling pathway.
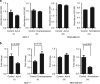
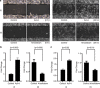
Results: We report that activin-A signaling is hyperactivated in breast cancers as indicated by higher activin-A, phosphoSMAD2, and phosphoSMAD3 levels in advanced breast cancers. Bone morphogenetic proteins and molecules involved in this signaling pathway were downregulated, suggesting its suppression in breast cancers. Activin-A expression correlates inversely with survival and metastasis in advanced breast cancers. Further, activin-A promotes anchorage-independent growth, epithelial-mesenchymal transition, invasion, angiogenesis, and stemness of breast cancer cells. We show that activin-A-induced phenotype is mediated by SMAD signaling pathway. In addition, activin-A expression affects the tumor-forming ability and metastatic colonization of cancer cells in nude mice.
Conclusions: These results suggest that activin-A has a critical role in breast cancer progression and, hence, targeting this pathway can be a valuable strategy in treating breast cancer patients.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Rivier C , Vale W , Rivier J . Studies of the inhibin family of hormones: a review. Horm Res 1987; 28: 104–118. - PubMed
-
- Tsuchida K , Nakatani M , Uezumi A , Murakami T , Cui X . Signal transduction pathway through activin receptors as a therapeutic target of musculoskeletal diseases and cancer. Endocr J 2008; 55: 11–21. - PubMed
-
- Ling N , Ying SY , Ueno N , Shimasaki S , Esch F , Hotta M et al. Pituitary FSH is released by a heterodimer of the beta-subunits from the two forms of inhibin. Nature 1986; 321: 779–782. - PubMed
-
- Vale W , Rivier J , Vaughan J , McClintock R , Corrigan A , Woo W et al. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature 1986; 321: 776–779. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

