Randomized trial of medroxyprogesterone acetate for the prevention of endometrial pathology from adjuvant tamoxifen for breast cancer: SWOG S9630
- PMID: 28721383
- PMCID: PMC5515330
- DOI: 10.1038/npjbcancer.2016.24
Randomized trial of medroxyprogesterone acetate for the prevention of endometrial pathology from adjuvant tamoxifen for breast cancer: SWOG S9630
Abstract
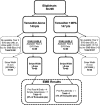
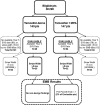
The proliferative effect of adjuvant tamoxifen on the endometrium can potentially result in endometrial abnormalities, including cancer in postmenopausal women. We conducted a randomized, controlled trial to assess endometrial pathological diagnoses in postmenopausal women with early stage, ER-positive breast cancer without endometrial pathology at baseline. They were assigned to tamoxifen alone versus tamoxifen plus cyclical medroxyprogesterone acetate (MPA 10 mg for 14 days every 3 months) for 5 years. Endovaginal sonograms (EVS) +/- endometrial biopsies (EMB) were required at baseline, 2 and 5 years. Of 313 patients registered, 296 were eligible and 169 (57%; 89, tamoxifen; 80, tamoxifen+MPA) were evaluable (completed year-2 EVS, with an EMB if stripe width was ⩾5 mm). Sixty (67%) of these in the tamoxifen arm had an endometrial stripe width ⩾5 mm (and underwent subsequent EMB) compared with 48 (60%) in the tamoxifen+MPA arm (P=0.40). There were four cases of proliferative endometrium and one simple hyperplasia on the tamoxifen arm (6% (95% confidence interval (CI): 2-13%) among evaluable patients and one proliferative endometrium on the tamoxifen+MPA arm (P=0.11). The overall fraction with benign endometrial abnormalities at year 2 was 3.6% (6/169; 95% CI: 1.3-7.6%), with only 1 (of 102) new benign proliferative event at year 5. The event rate in both arms was much lower than projected, making treatment arm comparisons less informative. A normal endometrium prior to tamoxifen may provide reassurance regarding future endometrial events. However, validation in a larger trial is needed before changing practice in asymptomatic, postmenopausal women.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 351, 1451–1467 (1998). - PubMed
-
- Fisher, B. et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet 353, 1993–2000 (1999). - PubMed
-
- Fisher, B. et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl Cancer Inst. 90, 1371–1388 (1998). - PubMed
-
- Killackey, M.A., Hakes, T.B. & Pierce, V.K. Endometrial adenocarcinoma in breast cancer patients receiving antiestrogens. Cancer Treat. Rep. 69, 237–238 (1985). - PubMed
-
- van Leeuwen, F.E. et al. Risk of endometrial cancer after tamoxifen treatment of breast cancer. Lancet 343, 448–452 (1994). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources