Human iPSC-Derived Cardiomyocytes for Investigation of Disease Mechanisms and Therapeutic Strategies in Inherited Arrhythmia Syndromes: Strengths and Limitations
- PMID: 28721524
- PMCID: PMC5550530
- DOI: 10.1007/s10557-017-6735-0
Human iPSC-Derived Cardiomyocytes for Investigation of Disease Mechanisms and Therapeutic Strategies in Inherited Arrhythmia Syndromes: Strengths and Limitations
Abstract
During the last two decades, significant progress has been made in the identification of genetic defects underlying inherited arrhythmia syndromes, which has provided some clinical benefit through elucidation of gene-specific arrhythmia triggers and treatment. However, for most arrhythmia syndromes, clinical management is hindered by insufficient knowledge of the functional consequences of the mutation in question, the pro-arrhythmic mechanisms involved, and hence the most optimal treatment strategy. Moreover, disease expressivity and sensitivity to therapeutic interventions often varies between mutations and/or patients, underlining the need for more individualized strategies. The development of the induced pluripotent stem cell (iPSC) technology now provides the opportunity for generating iPSC-derived cardiomyocytes (CMs) from human material (hiPSC-CMs), enabling patient- and/or mutation-specific investigations. These hiPSC-CMs may furthermore be employed for identification and assessment of novel therapeutic strategies for arrhythmia syndromes. However, due to their relative immaturity, hiPSC-CMs also display a number of essential differences as compared to adult human CMs, and hence there are certain limitations in their use. We here review the electrophysiological characteristics of hiPSC-CMs, their use for investigating inherited arrhythmia syndromes, and their applicability for identification and assessment of (novel) anti-arrhythmic treatment strategies.
Keywords: Arrhythmias; Cardiomyocytes; Human; Induced pluripotent stem cells; Pharmacology.
Conflict of interest statement
Funding
This work was funded in part by a Priority Medicines Rare Diseases and Orphan Drugs grant (PM-Rare, 113303006) from The Netherlands Organization for Health Research and Development (ZonMw) and an Innovational Research Incentives Scheme Vidi grant from ZonMw (grant no. 91714371, to Dr. Remme).
Conflict of Interest
Simona Casini declares that she has no conflict of interest, Arie O. Verkerk declares that he has no conflict of interest, and Carol Ann Remme has previously received a research grant from Gilead Sciences.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent Statement
Not applicable.
Figures
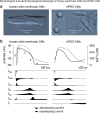

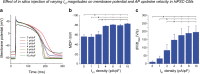



References
-
- Olde Nordkamp LR, Postema PG, Knops RE, van Dijk N, Limpens J, Wilde AA, et al. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: a systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm. 2016;13:443–454. doi: 10.1016/j.hrthm.2015.09.010. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

