Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials
- PMID: 28721545
- PMCID: PMC5515721
- DOI: 10.1186/s10194-017-0776-4
Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials
Abstract
Background: Mainly based on evidence of success in adults, various medications are commonly used to prevent pediatric migraines. Topiramate has been approved for migraine prevention in children as young as 12 years of age. In this meta-analysis, we aimed to assess the currently published data pertaining to the efficacy of topiramate for migraine prevention in patients less than 18 years of age.
Methods: We searched PubMed/Medline, Embase and the Cochrane Library (from inception to April 2017) for randomized controlled trials (RCTs) published in English. Two independent investigators performed data extraction and quality evaluation using the Cochrane Collaboration's tool. The data extracted were analyzed by Review Manager 5.3 software.
Results: A total of four RCTs matching the inclusion criteria were included, with an aggregate of 465 patients. Of these patients, 329 were included in the topiramate group, and 136 were included in the placebo group. This meta-analysis revealed that compared with placebo, topiramate failed to decrease the number of patients experiencing a ≥ 50% relative reduction in headache frequency (n = 465, RR = 1.26, 95% CI = [0.94,1.67], Z = 1.55, P = 0.12) or the number of headache days (n = 465, MD = -0.77, 95% CI = [-2.31,0.76], Z = 0.99, P = 0.32) but did reduce PedMIDAS scores (n = 205, MD = -9.02, 95% CI = [-17.34, -0.70], Z = 2.13, P = 0.03). Higher rates of side effects and adverse events in the topiramate group than in the placebo group were observed in the included trials.
Conclusions: Topiramate may not achieve a more effective clinical trial endpoint than placebo in the prevention of migraines in patients less than 18 years of age, and topiramate may lead to more side effects or adverse events in the included patients.
Keywords: Adolescent; Children; Migraine; Pediatric; Prevention; Topiramate.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

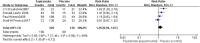
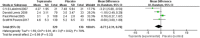


Comment in
-
PURL: Treating migraines: It's different for kids.J Fam Pract. 2018 Apr;67(4):238;239;241. J Fam Pract. 2018. PMID: 29614145 Free PMC article.
References
-
- Lewis D, Ashwal S, Hershey A, Hirtz D, Yonker M, Silberstein S. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology quality standards subcommittee and the practice Committee of the Child Neurology Society. Neurology. 2004;63(12):2215–2224. doi: 10.1212/01.WNL.0000147332.41993.90. - DOI - PubMed
-
- Lewis DW, Avener M, Gozzo Y. Pediatric migraine, part 1: update on classification, diagnosis, and the evaluation. Headache Pain Diagn Challenges Curr Ther. 2005;16(2):81–88.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

