Activation of the GABAergic Parafacial Zone Maintains Sleep and Counteracts the Wake-Promoting Action of the Psychostimulants Armodafinil and Caffeine
- PMID: 28722021
- PMCID: PMC5729562
- DOI: 10.1038/npp.2017.152
Activation of the GABAergic Parafacial Zone Maintains Sleep and Counteracts the Wake-Promoting Action of the Psychostimulants Armodafinil and Caffeine
Abstract
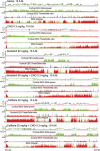
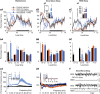
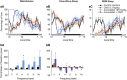
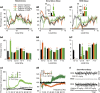
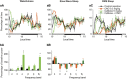
We previously reported that acute and selective activation of GABA-releasing parafacial zone (PZVgat) neurons in behaving mice produces slow-wave-sleep (SWS), even in the absence of sleep deficit, suggesting that these neurons may represent, at least in part, a key cellular substrate underlying sleep drive. It remains, however, to be determined if PZVgat neurons actively maintain, as oppose to simply gate, SWS. To begin to experimentally address this knowledge gap, we asked whether activation of PZVgat neurons could attenuate or block the wake-promoting effects of two widely used wake-promoting psychostimulants, armodafinil or caffeine. We found that activation of PZVgat neurons completely blocked the behavioral and electrocortical wake-promoting action of armodafinil. In some contrast, activation of PZVgat neurons inhibited the behavioral, but not electrocortical, arousal response to caffeine. These results suggest that: (1) PZVgat neurons actively maintain, as oppose to simply gate, SWS and cortical slow-wave-activity; (2) armodafinil cannot exert its wake-promoting effects when PZVgat neurons are activated, intimating a possible shared circuit/molecular basis for mechanism of action; (3) caffeine can continue to exert potent cortical desynchronizing, but not behavioral, effects when PZVgat neurons are activated, inferring a shared and divergent circuit/molecular basis for mechanism of action; and 4) PZVgat neurons represent a key cell population for SWS induction and maintenance.
Figures
References
-
- Basheer R, Rainnie DG, Porkka-Heiskanen T, Ramesh V, McCarley RW (2001). Adenosine, prolonged wakefulness, and A1-activated NF-kappaB DNA binding in the basal forebrain of the rat. Neuroscience 104: 731–739. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

