Opioid Release after High-Intensity Interval Training in Healthy Human Subjects
- PMID: 28722022
- PMCID: PMC5729560
- DOI: 10.1038/npp.2017.148
Opioid Release after High-Intensity Interval Training in Healthy Human Subjects
Abstract
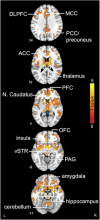
Central opioidergic mechanisms may modulate the positive effects of physical exercise such as mood elevation and stress reduction. How exercise intensity and concomitant effective changes affect central opioidergic responses is unknown. We studied the effects of acute physical exercise on the cerebral μ-opioid receptors (MOR) of 22 healthy recreationally active males using positron emission tomography (PET) and the MOR-selective radioligand [11C]carfentanil. MOR binding was measured in three conditions on separate days: after a 60-min aerobic moderate-intensity exercise session, after a high-intensity interval training (HIIT) session, and after rest. Mood was measured repeatedly throughout the experiment. HIIT significantly decreased MOR binding selectively in the frontolimbic regions involved in pain, reward, and emotional processing (thalamus, insula, orbitofrontal cortex, hippocampus, and anterior cingulate cortex). Decreased binding correlated with increased negative emotionality. Moderate-intensity exercise did not change MOR binding, although increased euphoria correlated with decreased receptor binding. These observations, consistent with endogenous opioid release, highlight the role of the μ-opioid system in mediating affective responses to high-intensity training as opposed to recreational moderate physical exercise.
Figures



Similar articles
-
Aerobic exercise modulates anticipatory reward processing via the μ-opioid receptor system.Hum Brain Mapp. 2018 Oct;39(10):3972-3983. doi: 10.1002/hbm.24224. Epub 2018 Jun 8. Hum Brain Mapp. 2018. PMID: 29885086 Free PMC article.
-
Aerobic Fitness Is Associated with Cerebral μ-Opioid Receptor Activation in Healthy Humans.Med Sci Sports Exerc. 2022 Jul 1;54(7):1076-1084. doi: 10.1249/MSS.0000000000002895. Epub 2022 Feb 23. Med Sci Sports Exerc. 2022. PMID: 35195103
-
Altered central micro-opioid receptor binding after psychological trauma.Biol Psychiatry. 2007 May 1;61(9):1030-8. doi: 10.1016/j.biopsych.2006.06.021. Epub 2006 Aug 30. Biol Psychiatry. 2007. PMID: 16945349
-
What to learn from in vivo opioidergic brain imaging?Eur J Pain. 2005 Apr;9(2):117-21. doi: 10.1016/j.ejpain.2004.07.010. Eur J Pain. 2005. PMID: 15737798 Review.
-
Measuring our natural painkiller.Trends Neurosci. 2002 Feb;25(2):67-8; discussion 69. doi: 10.1016/s0166-2236(02)02025-8. Trends Neurosci. 2002. PMID: 11814550 Review.
Cited by
-
A hominid-specific shift in cerebellar expression, upstream retrotransposons, and a potential cis-regulatory mechanism: bioinformatics analyses of the mu-opioid receptor gene.Heredity (Edinb). 2020 Feb;124(2):325-335. doi: 10.1038/s41437-019-0282-3. Epub 2019 Nov 11. Heredity (Edinb). 2020. PMID: 31712748 Free PMC article.
-
Athlete level, sport-type, and gender influences on training, mental health, and sleep during the early COVID-19 lockdown in Malaysia.Front Physiol. 2023 Jan 11;13:1093965. doi: 10.3389/fphys.2022.1093965. eCollection 2022. Front Physiol. 2023. PMID: 36714309 Free PMC article.
-
Lowered endogenous mu-opioid receptor availability in subclinical depression and anxiety.Neuropsychopharmacology. 2020 Oct;45(11):1953-1959. doi: 10.1038/s41386-020-0725-9. Epub 2020 May 30. Neuropsychopharmacology. 2020. PMID: 32473595 Free PMC article.
-
Modulation of pain perceptions following treadmill running with different intensities in females.Physiol Rep. 2023 Sep;11(18):e15831. doi: 10.14814/phy2.15831. Physiol Rep. 2023. PMID: 37749050 Free PMC article. Clinical Trial.
-
High-intensity interval training in the prehabilitation of cancer patients-a systematic review and meta-analysis.Support Care Cancer. 2021 Apr;29(4):1781-1794. doi: 10.1007/s00520-020-05834-x. Epub 2020 Oct 26. Support Care Cancer. 2021. PMID: 33106975 Free PMC article.
References
-
- Aaltonen S, Leskinen T, Morris T, Alen M, Kaprio J, Liukkonen J et al (2012). Motives for and barriers to physical activity in twin pairs discordant for leisure time physical activity for 30 years. Int J Sports Med 33: 157–163. - PubMed
-
- Arida RM, Gomes da Silva S, de Almeida AA, Cavalheiro EA, Zavala-Tecuapetla C, Brand S et al (2015). Differential effects of exercise on brain opioid receptor binding and activation in rats. J Neurochem 132: 206–217. - PubMed
-
- Bartlett JD, Close GL, MacLaren DPM, Gregson W, Drust B, Morton JP (2011). High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: implications for exercise adherence. J Sports Sci 29: 547–553. - PubMed
-
- Boecker H, Sprenger T, Spilker ME, Henriksen G, Koppenhoefer M, Wagner KJ et al (2008). The runner’s high: opioidergic mechanisms in the human brain. Cereb Cortex 18: 2523–2531. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

