Phase 2a, Open-Label, 4-Escalating-Dose, Randomized Multicenter Study Evaluating the Safety and Activity of Ferroquine (SSR97193) Plus Artesunate, versus Amodiaquine Plus Artesunate, in African Adult Men with Uncomplicated Plasmodium falciparum Malaria
- PMID: 28722611
- PMCID: PMC5544076
- DOI: 10.4269/ajtmh.16-0731
Phase 2a, Open-Label, 4-Escalating-Dose, Randomized Multicenter Study Evaluating the Safety and Activity of Ferroquine (SSR97193) Plus Artesunate, versus Amodiaquine Plus Artesunate, in African Adult Men with Uncomplicated Plasmodium falciparum Malaria
Abstract
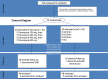
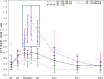
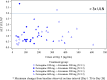
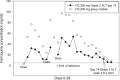
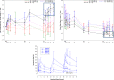
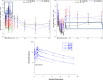
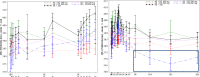
Artemisinin-based combination therapies are recommended as first-line agents for treating uncomplicated Plasmodium falciparum malaria. Ferroquine, a 4-aminoquinolone, is a novel long-acting combination partner for fast-acting drugs like artesunate (AS). We did a small phase 2a, multicenter, open-label, safety-focused dose-ranging randomized study of ferroquine at three African hospitals: two Gabonese and one Kenyan. We recruited adult men with symptomatic uncomplicated P. falciparum monoinfection. Four escalating doses of ferroquine (100, 200, 400, and 600 mg) were assessed in sequence, versus an amodiaquine comparator. After a 2:1 randomization (block size three, equating to N = 12 for each ferroquine dose and N = 6 for each of four amodiaquine comparator groups) patients received daily for three consecutive days, either ferroquine + AS (200 mg/day) or amodiaquine (612 mg/day) + AS (200 mg/day). Safety, electrocardiograms, parasite clearance times, efficacy, and pharmacokinetics were assessed to day 28. Seventy-two patients were randomized. Ferroquine + AS showed generally mild increases (Grade 1 toxicity) in alanine aminotransferase (ALT) levels with a dose trend starting at 400 mg. There were two Grade 2 ALT events: one patient receiving 200 mg (3.8 upper limit of normal [ULN], day 7) and one receiving 600 mg (3.3 ULN, day 14), both without increased bilirubin. One ferroquine 100 mg + AS patient after one dose was withdrawn after developing a QTcF interval prolongation > 60 milliseconds over baseline. Parasitemias in all patients cleared quickly, with no recurrence through day 28. Hepatic, as well as cardiac, profiles should be monitored closely in future trials. (ClinicalTrials.gov: NCT00563914).
Conflict of interest statement
Conflicts of interest: Cathy Cantalloube and Elhadj Djeriou are employees of Sanofi-Aventis. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors may consider relevant to the content of the manuscript have been disclosed.
Figures



Comment in
-
Ferroquine Advances.Am J Trop Med Hyg. 2017 Aug;97(2):309-310. doi: 10.4269/ajtmh.17-0373. Am J Trop Med Hyg. 2017. PMID: 28829727 Free PMC article. No abstract available.
References
-
- Hawkes M, Conroy AL, Kain KC, 2014. Spread of artemisinin resistance in malaria. N Engl J Med 371: 1944–1945. - PubMed
-
- Atteke C, Ndong JM, Aubouy A, Maciejewski L, Brocard J, Lébibi J, Deloron P, 2003. In vitro susceptibility to a new antimalarial organometallic analogue, ferroquine, of Plasmodium falciparum isolates from the Haut-Ogooue region of Gabon. J Antimicrob Chemother 51: 1021–1024. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

