Enhanced Prophylaxis plus Antiretroviral Therapy for Advanced HIV Infection in Africa
- PMID: 28723333
- PMCID: PMC5603269
- DOI: 10.1056/NEJMoa1615822
Enhanced Prophylaxis plus Antiretroviral Therapy for Advanced HIV Infection in Africa
Abstract
Background: In sub-Saharan Africa, among patients with advanced human immunodeficiency virus (HIV) infection, the rate of death from infection (including tuberculosis and cryptococcus) shortly after the initiation of antiretroviral therapy (ART) is approximately 10%.
Methods: In this factorial open-label trial conducted in Uganda, Zimbabwe, Malawi, and Kenya, we enrolled HIV-infected adults and children 5 years of age or older who had not received previous ART and were starting ART with a CD4+ count of fewer than 100 cells per cubic millimeter. They underwent simultaneous randomization to receive enhanced antimicrobial prophylaxis or standard prophylaxis, adjunctive raltegravir or no raltegravir, and supplementary food or no supplementary food. Here, we report on the effects of enhanced antimicrobial prophylaxis, which consisted of continuous trimethoprim-sulfamethoxazole plus at least 12 weeks of isoniazid-pyridoxine (coformulated with trimethoprim-sulfamethoxazole in a single fixed-dose combination tablet), 12 weeks of fluconazole, 5 days of azithromycin, and a single dose of albendazole, as compared with standard prophylaxis (trimethoprim-sulfamethoxazole alone). The primary end point was 24-week mortality.
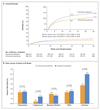
Results: A total of 1805 patients (1733 adults and 72 children or adolescents) underwent randomization to receive either enhanced prophylaxis (906 patients) or standard prophylaxis (899 patients) and were followed for 48 weeks (loss to follow-up, 3.1%). The median baseline CD4+ count was 37 cells per cubic millimeter, but 854 patients (47.3%) were asymptomatic or mildly symptomatic. In the Kaplan-Meier analysis at 24 weeks, the rate of death with enhanced prophylaxis was lower than that with standard prophylaxis (80 patients [8.9% vs. 108 [12.2%]; hazard ratio, 0.73; 95% confidence interval [CI], 0.55 to 0.98; P=0.03); 98 patients (11.0%) and 127 (14.4%), respectively, had died by 48 weeks (hazard ratio, 0.76; 95% CI, 0.58 to 0.99; P=0.04). Patients in the enhanced-prophylaxis group had significantly lower rates of tuberculosis (P=0.02), cryptococcal infection (P=0.01), oral or esophageal candidiasis (P=0.02), death of unknown cause (P=0.03), and new hospitalization (P=0.03). However, there was no significant between-group difference in the rate of severe bacterial infection (P=0.32). There were nonsignificantly lower rates of serious adverse events and grade 4 adverse events in the enhanced-prophylaxis group (P=0.08 and P=0.09, respectively). Rates of HIV viral suppression and adherence to ART were similar in the two groups.
Conclusions: Among HIV-infected patients with advanced immunosuppression, enhanced antimicrobial prophylaxis combined with ART resulted in reduced rates of death at both 24 weeks and 48 weeks without compromising viral suppression or increasing toxic effects. (Funded by the Medical Research Council and others; REALITY Current Controlled Trials number, ISRCTN43622374 .).
Conflict of interest statement
Dr. Hakim reports receiving fees for serving on an advisory board from Mylan Pharmaceuticals and consulting fees from Gilead Sciences and Johnson & Johnson; Dr. Prendergast, receiving fees for preparing educational material from the PENTA Foundation; and Dr. A. Walker, receiving fees paid to her institution for serving on a data and safety monitoring board for Tibotec and lecture fees paid to her institution by Gilead Sciences. No other potential conflict of interest relevant to this article was reported.
Figures


Comment in
-
The Enduring Challenge of Advanced HIV Infection.N Engl J Med. 2017 Jul 20;377(3):283-284. doi: 10.1056/NEJMe1707598. N Engl J Med. 2017. PMID: 28723319 No abstract available.
-
Supplementary antimicrobials for patients with HIV and <100 CD4 cells/µL are associated with improved survival.Evid Based Med. 2017 Dec;22(6):209. doi: 10.1136/ebmed-2017-110843. Epub 2017 Nov 13. Evid Based Med. 2017. PMID: 29133303 No abstract available.
References
-
- Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd ed. Geneva: World Health Organization; 2016. ( http://www.who.int/hiv/pub/arv/arv-2016/en/) - PubMed
-
- The TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808–22. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
