Association of Implantable Cardioverter Defibrillators With Survival in Patients With and Without Improved Ejection Fraction: Secondary Analysis of the Sudden Cardiac Death in Heart Failure Trial
- PMID: 28724134
- PMCID: PMC5710619
- DOI: 10.1001/jamacardio.2017.1413
Association of Implantable Cardioverter Defibrillators With Survival in Patients With and Without Improved Ejection Fraction: Secondary Analysis of the Sudden Cardiac Death in Heart Failure Trial
Abstract
Importance: Improvement in left ventricular ejection fraction (EF) to >35% occurs in many patients with reduced EF at baseline. To our knowledge, whether implantable cardioverter defibrillator (ICD) therapy improves survival for these patients is unknown.
Objective: To examine the efficacy of ICD therapy in reducing risk of all-cause mortality and sudden cardiac death among patients with an EF ≤35% at baseline, with or without an improvement in EF to >35% during follow-up.
Design, setting, and participants: This retrospective analysis examined data collected in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT), which randomly assigned 2521 patients to placebo, amiodarone, or ICD between 1997 and 2001. A subset of 1902 participants (75.4%) of the SCD-HeFT had a repeated assessment of EF a mean (SD) of 13.5 (6) months after randomization. We stratified these patients by EF ≤35% and >35% based on the first repeated EF measurement after randomization and compared all-cause mortality in 649 patients randomized to placebo vs 624 patients randomized to ICD. Follow-up started with the repeated EF assessment. Analysis was performed between January 2016 and July 2016.
Exposures: Implantable cardioverter-defibrillator therapy.
Main outcomes and measures: All-cause mortality and sudden cardiac death.
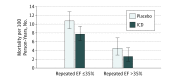
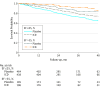
Results: Of the included 1273 patients, the mean (SD) age was 59 (12) years, and 977 (76.7%) were male and 1009 (79.3%) were white. Repeated EF was >35% in 186 participants (29.8%) randomized to ICD and 185 participants (28.5%) randomized to placebo. During a median follow-up of 30 months, the all-cause mortality rate was lower in the ICD vs placebo group, both in patients whose EF remained ≤35% (7.7 vs 10.7 per 100 person-year follow-up) and in those whose EF improved to >35% (2.6 vs 4.5 per 100 person-year follow-up). Compared with placebo, the adjusted hazard ratio for the effect of ICD on mortality was 0.64 (95% CI, 0.48-0.85) in patients with a repeated EF of ≤35% and 0.62 (95% CI, 0.29-1.30) in those with a repeated EF >35%. There was no interaction between treatment assignment and repeated EF for predicting mortality.
Conclusions and relevance: Among participants in the SCD-HeFT who had a repeated EF assessment during the course of follow-up, those who had an improvement in EF to >35% accrued a similar relative reduction in mortality with ICD therapy as those whose EF remained ≤35%. Prospective randomized clinical trials are needed to test ICD efficacy in patients with an EF >35%.
Trial registration: clinicaltrials.gov Identifier: NCT01114269.
Conflict of interest statement
Figures
Comment in
-
Benefits of Contemporary Implantable Cardioverter Defibrillators in Patients With Improved Ejection Fraction: When is the Most Clinically Relevant Time to Evaluate?-Reply.JAMA Cardiol. 2017 Dec 1;2(12):1397-1398. doi: 10.1001/jamacardio.2017.3458. JAMA Cardiol. 2017. PMID: 28973098 No abstract available.
-
Benefits of Contemporary Implantable Cardioverter Defibrillators in Patients With Improved Ejection Fraction: When Is the Most Clinically Relevant Time to Evaluate?JAMA Cardiol. 2017 Dec 1;2(12):1397. doi: 10.1001/jamacardio.2017.3448. JAMA Cardiol. 2017. PMID: 28973138 No abstract available.
References
-
- Bardy GH, Lee KL, Mark DB, et al. ; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators . Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225-237. - PubMed
-
- Moss AJ, Zareba W, Hall WJ, et al. ; Multicenter Automatic Defibrillator Implantation Trial II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877-883. - PubMed
-
- Myerburg RJ, Reddy V, Castellanos A. Indications for implantable cardioverter-defibrillators based on evidence and judgment. J Am Coll Cardiol. 2009;54(9):747-763. - PubMed
-
- Epstein AE, DiMarco JP, Ellenbogen KA, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society . 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61(3):e6-e75. - PubMed
-
- Punnoose LR, Givertz MM, Lewis EF, Pratibhu P, Stevenson LW, Desai AS. Heart failure with recovered ejection fraction: a distinct clinical entity. J Card Fail. 2011;17(7):527-532. - PubMed
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

