A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma
- PMID: 28724573
- PMCID: PMC5762203
- DOI: 10.1126/scitranslmed.aaa0984
A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma
Abstract
We conducted a first-in-human study of intravenous delivery of a single dose of autologous T cells redirected to the epidermal growth factor receptor variant III (EGFRvIII) mutation by a chimeric antigen receptor (CAR). We report our findings on the first 10 recurrent glioblastoma (GBM) patients treated. We found that manufacturing and infusion of CAR-modified T cell (CART)-EGFRvIII cells are feasible and safe, without evidence of off-tumor toxicity or cytokine release syndrome. One patient has had residual stable disease for over 18 months of follow-up. All patients demonstrated detectable transient expansion of CART-EGFRvIII cells in peripheral blood. Seven patients had post-CART-EGFRvIII surgical intervention, which allowed for tissue-specific analysis of CART-EGFRvIII trafficking to the tumor, phenotyping of tumor-infiltrating T cells and the tumor microenvironment in situ, and analysis of post-therapy EGFRvIII target antigen expression. Imaging findings after CART immunotherapy were complex to interpret, further reinforcing the need for pathologic sampling in infused patients. We found trafficking of CART-EGFRvIII cells to regions of active GBM, with antigen decrease in five of these seven patients. In situ evaluation of the tumor environment demonstrated increased and robust expression of inhibitory molecules and infiltration by regulatory T cells after CART-EGFRvIII infusion, compared to pre-CART-EGFRvIII infusion tumor specimens. Our initial experience with CAR T cells in recurrent GBM suggests that although intravenous infusion results in on-target activity in the brain, overcoming the adaptive changes in the local tumor microenvironment and addressing the antigen heterogeneity may improve the efficacy of EGFRvIII-directed strategies in GBM.
Copyright © 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
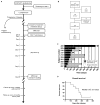
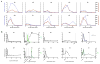
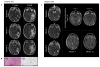
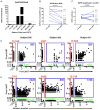

Comment in
-
Immunotherapy: Adaptive resistance to CARs in glioma.Nat Rev Clin Oncol. 2017 Oct;14(10):586-587. doi: 10.1038/nrclinonc.2017.126. Epub 2017 Aug 8. Nat Rev Clin Oncol. 2017. PMID: 28786418 No abstract available.
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Sathornsumetee S, Reardon DA. Targeting multiple kinases in glioblastoma multiforme. Expert Opin Investig Drugs. 2009;18:277–292. - PubMed
-
- Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, Taylor LP, Lieberman F, Silvani A, Fink KL, Barnett GH, Zhu JJ, Henson JW, Engelhard HH, Chen TC, Tran DD, Sroubek J, Tran ND, Hottinger AF, Landolfi J, Desai R, Caroli M, Kew Y, Honnorat J, Idbaih A, Kirson ED, Weinberg U, Palti Y, Hegi ME, Ram Z. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: A randomized clinical trial. JAMA. 2015;314:2535–2543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

