Transcription Factor MYB26 Is Key to Spatial Specificity in Anther Secondary Thickening Formation
- PMID: 28724622
- PMCID: PMC5580765
- DOI: 10.1104/pp.17.00719
Transcription Factor MYB26 Is Key to Spatial Specificity in Anther Secondary Thickening Formation
Abstract
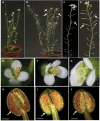
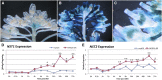
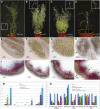
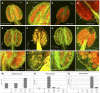
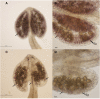
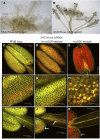
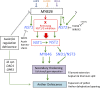
Successful fertilization relies on the production and effective release of viable pollen. Failure of anther opening (dehiscence), results in male sterility, although the pollen may be fully functional. MYB26 regulates the formation of secondary thickening in the anther endothecium, which is critical for anther dehiscence and fertility. Here, we show that although the MYB26 transcript shows expression in multiple floral organs, the MYB26 protein is localized specifically to the anther endothecium nuclei and that it directly regulates two NAC domain genes, NST1 and NST2, which are critical for the induction of secondary thickening biosynthesis genes. However, there is a complex relationship of regulation between these genes and MYB26. Using DEX-inducible MYB26 lines and overexpression in the various mutant backgrounds, we have shown that MYB26 up-regulates both NST1 and NST2 expression. Surprisingly normal thickening and fertility rescue does not occur in the absence of MYB26, even with constitutively induced NST1 and NST2, suggesting an additional essential role for MYB26 in this regulation. Combined overexpression of NST1 and NST2 in myb26 facilitates limited ectopic thickening in the anther epidermis, but not in the endothecium, and thus fails to rescue dehiscence. Therefore, by a series of regulatory controls through MYB26, NST1, NST2, secondary thickening is formed specifically within the endothecium; this specificity is essential for anther opening.
© 2017 American Society of Plant Biologists. All Rights Reserved.
Figures


References
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Ariizumi T, Toriyama K (2011) Genetic regulation of sporopollenin synthesis and pollen exine development. Annu Rev Plant Biol 62: 437–460 - PubMed
-
- Caffall KH, Pattathil S, Phillips SE, Hahn MG, Mohnen D (2009) Arabidopsis thaliana T-DNA mutants implicate GAUT genes in the biosynthesis of pectin and xylan in cell walls and seed testa. Mol Plant 2: 1000–1014 - PubMed
-
- Cecchetti V, Altamura MM, Brunetti P, Petrocelli V, Falasca G, Ljung K, Costantino P, Cardarelli M (2013) Auxin controls Arabidopsis anther dehiscence by regulating endothecium lignification and jasmonic acid biosynthesis. Plant J 74: 411–422 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

