Heterodimers of the transcriptional factors NFATc3 and FosB mediate tissue factor expression for 15(S)-hydroxyeicosatetraenoic acid-induced monocyte trafficking
- PMID: 28724635
- PMCID: PMC5592668
- DOI: 10.1074/jbc.M117.804344
Heterodimers of the transcriptional factors NFATc3 and FosB mediate tissue factor expression for 15(S)-hydroxyeicosatetraenoic acid-induced monocyte trafficking
Erratum in
-
Correction: Heterodimers of the transcriptional factors NFATc3 and FosB mediate tissue factor expression for 15(S)-hydroxyeicosatetraenoic acid-induced monocyte trafficking.J Biol Chem. 2022 Apr;298(4):101812. doi: 10.1016/j.jbc.2022.101812. Epub 2022 Mar 15. J Biol Chem. 2022. PMID: 35303608 Free PMC article. No abstract available.
Abstract
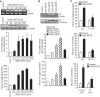
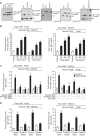
Tissue factor (TF) is expressed in vascular and nonvascular tissues and functions in several pathways, including embryonic development, inflammation, and cell migration. Many risk factors for atherosclerosis, including hypertension, diabetes, obesity, and smoking, increase TF expression. To better understand the TF-related mechanisms in atherosclerosis, here we investigated the role of 12/15-lipoxygenase (12/15-LOX) in TF expression. 15(S)-hydroxyeicosatetraenoic acid (15(S)-HETE), the major product of human 15-LOXs 1 and 2, induced TF expression and activity in a time-dependent manner in the human monocytic cell line THP1. Moreover, TF suppression with neutralizing antibodies blocked 15(S)-HETE-induced monocyte migration. We also found that NADPH- and xanthine oxidase-dependent reactive oxygen species (ROS) production, calcium/calmodulin-dependent protein kinase IV (CaMKIV) activation, and interactions between nuclear factor of activated T cells 3 (NFATc3) and FosB proto-oncogene, AP-1 transcription factor subunit (FosB) are involved in 15(S)-HETE-induced TF expression. Interestingly, NFATc3 first induced the expression of its interaction partner FosB before forming the heterodimeric NFATc3-FosB transcription factor complex, which bound the proximal AP-1 site in the TF gene promoter and activated TF expression. We also observed that macrophages from 12/15-LOX-/- mice exhibit diminished migratory response to monocyte chemotactic protein 1 (MCP-1) and lipopolysaccharide compared with WT mouse macrophages. Similarly, compared with WT macrophages, monocytes from 12/15-LOX-/- mice displayed diminished trafficking, which was rescued by prior treatment with 12(S)-HETE, in a peritonitis model. These observations indicate that 15(S)-HETE-induced monocyte/macrophage migration and trafficking require ROS-mediated CaMKIV activation leading to formation of NFATc3 and FosB heterodimer, which binds and activates the TF promoter.
Keywords: gene expression; migration; monocyte; signal transduction; trafficking.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures








References
-
- Ruf W., and Edgington T. S. (1994) Structural biology of tissue factor, the initiator of thrombogenesis in vivo. FASEB J. 8, 385–390 - PubMed
-
- Pawlinski R., Fernandes A., Kehrle B., Pedersen B., Parry G., Erlich J., Pyo R., Gutstein D., Zhang J., Castellino F., Melis E., Carmeliet P., Baretton G., Luther T., Taubman M., Rosen E., and Mackman N. (2002) Tissue factor deficiency causes cardiac fibrosis and left ventricular dysfunction. Proc. Natl. Acad. Sci. U.S.A. 99, 15333–15338 - PMC - PubMed
-
- Nishibe T., Parry G., Ishida A., Aziz S., Murray J., Patel Y., Rahman S., Strand K., Saito K., Saito Y., Hammond W. P., Savidge G. F., Mackman N., and Wijelath E. S. (2001) Oncostatin M promotes biphasic tissue factor expression in smooth muscle cells: Evidence for Erk-1/2 activation. Blood 97, 692–699 - PubMed
-
- Chen J., Kasper M., Heck T., Nakagawa K., Humpert P. M., Bai L., Wu G., Zhang Y., Luther T., Andrassy M., Schiekofer S., Hamann A., Morcos M., Chen B., Stern D. M., Nawroth P. P., and Bierhaus A. (2005) Tissue factor as a link between wounding and tissue repair. Diabetes 54, 2143–2154 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

