Hyperkalemia After Initiating Renin-Angiotensin System Blockade: The Stockholm Creatinine Measurements (SCREAM) Project
- PMID: 28724651
- PMCID: PMC5586281
- DOI: 10.1161/JAHA.116.005428
Hyperkalemia After Initiating Renin-Angiotensin System Blockade: The Stockholm Creatinine Measurements (SCREAM) Project
Abstract
Background: Concerns about hyperkalemia limit the use of angiotensin-converting enzyme inhibitors (ACE-I) and angiotensin receptor blockers (ARBs), but guidelines conflict regarding potassium-monitoring protocols. We quantified hyperkalemia monitoring and risks after ACE-I/ARB initiation and developed and validated a hyperkalemia susceptibility score.
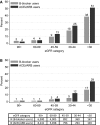
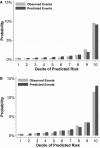
Methods and results: We evaluated 69 426 new users of ACE-I/ARB therapy in the Stockholm Creatinine Measurements (SCREAM) project with medication initiation from January 1, 2007 to December 31, 2010, and follow-up for 1 year thereafter. Three fourths (76%) of SCREAM patients had potassium checked within the first year. Potassium >5 and >5.5 mmol/L occurred in 5.6% and 1.7%, respectively. As a comparison, we propensity-matched new ACE-I/ARB users to 20 186 new β-blocker users in SCREAM: 64% had potassium checked. The occurrence of elevated potassium levels was similar between new β-blocker and ACE-I/ARB users without kidney disease; only at estimated glomerular filtration rate <60 mL/min per 1.73 m2 were risks higher among ACE-I/ARB users. We developed a hyperkalemia susceptibility score that incorporated estimated glomerular filtration rate, baseline potassium level, sex, diabetes mellitus, heart failure, and the concomitant use of potassium-sparing diuretics in new ACE-I/ARB users; this score accurately predicted 1-year hyperkalemia risk in the SCREAM cohort (area under the curve, 0.845, 95% CI: 0.840-0.869) and in a validation cohort from the US-based Geisinger Health System (N=19 524; area under the curve, 0.818, 95% CI: 0.794-0.841), with good calibration.
Conclusions: Hyperkalemia within the first year of ACE-I/ARB therapy was relatively uncommon among people with estimated glomerular filtration rate >60 mL/min per 1.73 m2, but rates were much higher with lower estimated glomerular filtration rate. Use of the hyperkalemia susceptibility score may help guide laboratory monitoring and prescribing strategies.
Keywords: angiotensin receptor blockers; angiotensin‐converting enzyme inhibition; angiotensin‐converting enzyme inhibitors; chronic kidney disease; hyperkalemia; potassium; risk score.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures
References
-
- Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, Ponticelli C, Ritz E, Zucchelli P. Effect of the angiotensin‐converting‐enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin‐Converting‐Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med. 1996;334:939–945. - PubMed
-
- Viberti G, Mogensen CE, Groop LC, Pauls JF. Effect of captopril on progression to clinical proteinuria in patients with insulin‐dependent diabetes mellitus and microalbuminuria. European Microalbuminuria Captopril Study Group. JAMA. 1994;271:275–279. - PubMed
-
- Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325:293–302. - PubMed
-
- Pfeffer MA, Lamas GA, Vaughan DE, Parisi AF, Braunwald E. Effect of captopril on progressive ventricular dilatation after anterior myocardial infarction. N Engl J Med. 1988;319:80–86. - PubMed
-
- The Heart Outcomes Prevention Evaluation Study Investigators . Effects of an angiotensin‐converting–enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. N Engl J Med. 2000;342:145–153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

